Nico Gagelmann: Current status and Future directions for CAR-T Therapy
Nico Gagelmann, Co-chair of EBMT’s Trainee Committee, shared a post on X:
“Chimeric antigen receptor T cell therapy might revolutionize the management and our understanding of autoimmune diseases An short educational thread on current status and future directions.
Intro:
– CAR T therapy was originally designed to fight cancer
– now showed transformative potential in treating autoimmune diseases by targeting B cells
key role in disease (progression)
– safety remains focus, with a milder toxicity profile emerging in autoimmune disease
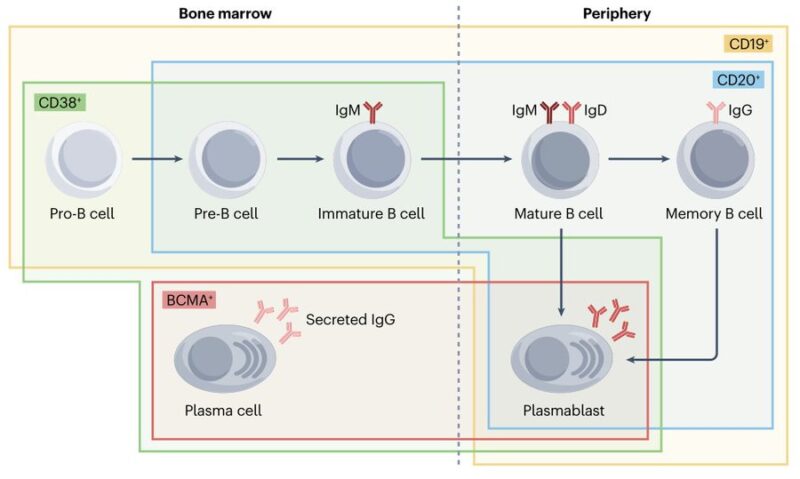
B cells?
– arise in bone marrow and go to periphery
– differentiate into antibody-secreting plasma cell populations
short-lived plasmablasts + long-lived plasma cells
reside in bone marrow
– CD19, CD20, CD38, BCMA expressed at various stages enable identification
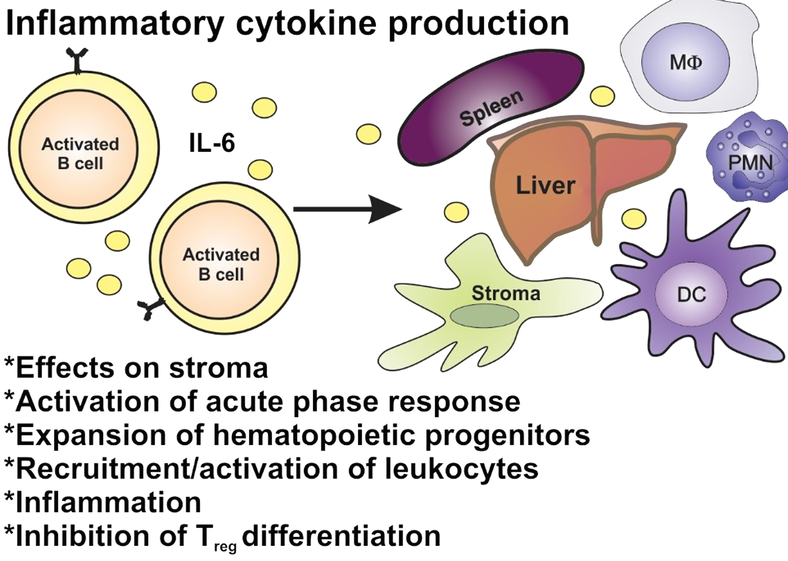
Targeting B cells:
– critical role not just by producing autoantibodies, through inflammatory cytokines, antigen presentation
– autoimmune conditions show defects in B cell tolerance checkpoints
causing autoreactive B cells to persist and contribute to disease progression
Treatments targeting B cells (anti-CD20 monoclonal antibodies, eg rituximab) have shown success in diseases like rheumatoid arthritis, multiple sclerosis, lupus nephritis
BUT
some B cells remain resilient
– tissue-resident cells in kidneys
– incomplete disease control
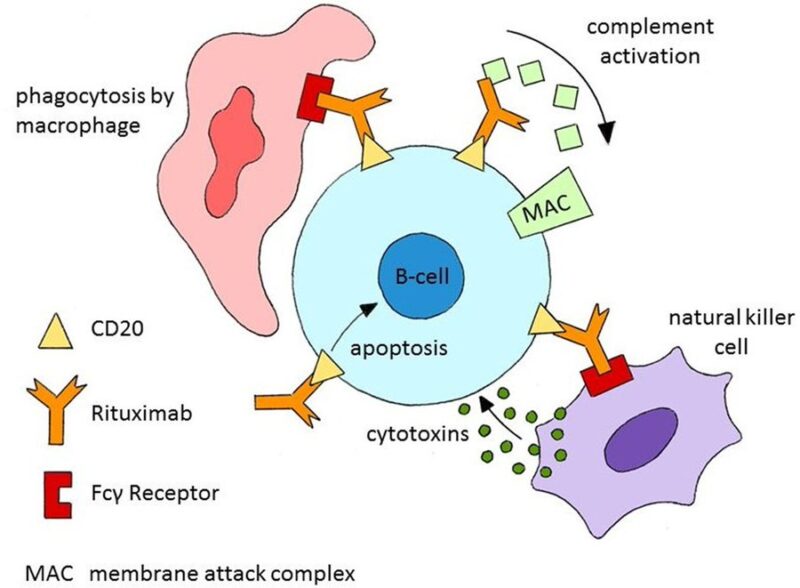
Different types of anti-CD20 antibodies use varied mechanisms for B cell depletion
– Type 1 (like rituximab)
complement-dependent cytotoxicity but has limited impact on tissue-resident B cells
– Type 2 (like obinutuzumab)
direct cell death
tackle resistant B cell subsets
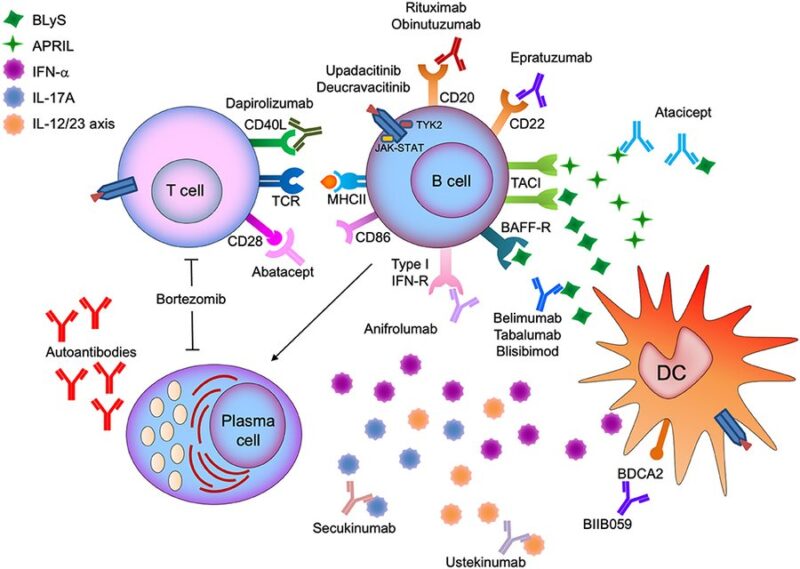
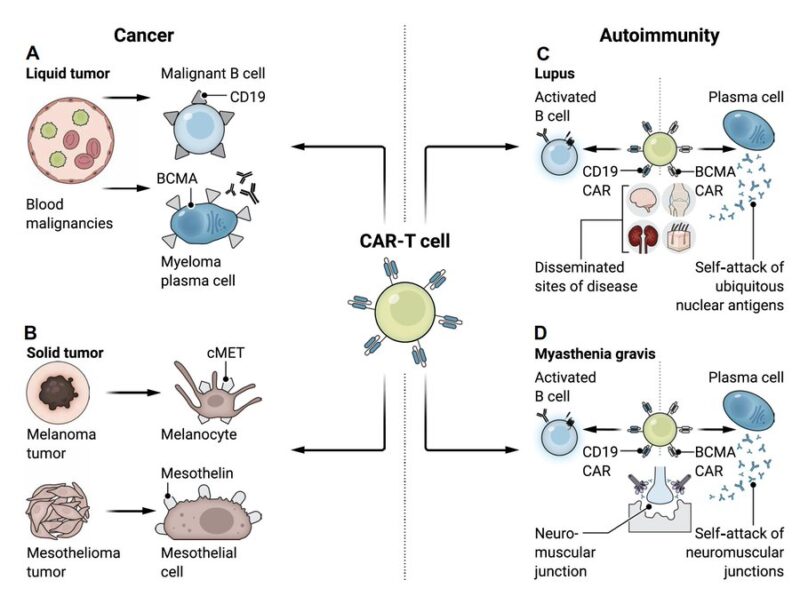
Benefit of CAR-T:
– targeting CD19 represents a new approach
offering deeper tissue-based B cell depletion
– in lupus, CAR T showed ability to target B cells in the spleen, inflamed kidneys, and bone marrow
to reduced autoantibodies and improved survival
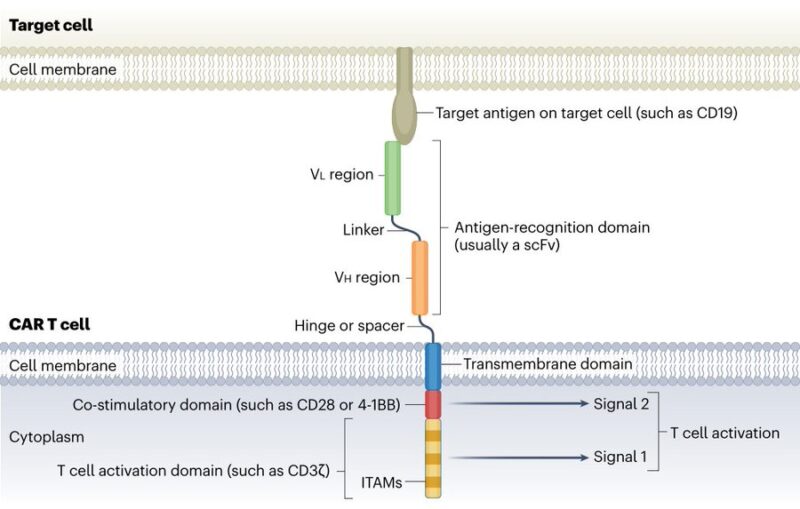
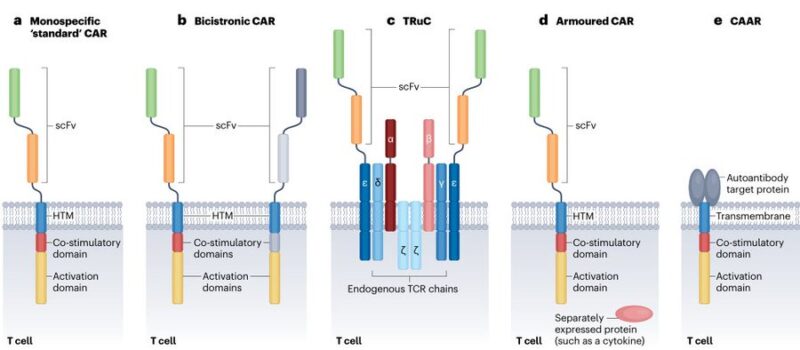
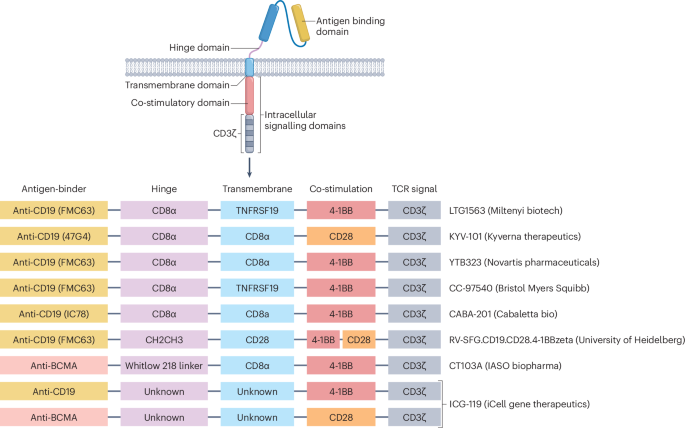
Possible CAR-T constructs:
– monospecific
standard CAR uses a scFv for antigen recognition, linked to a hinge and transmembrane domain, followed by co-stimulatory and T cell activation domains
– bicistronic
encodes 2 distinct CARs within a single T cell
– TCR Fusion Construct (TRuC)
fuses scFvs to a native T cell receptor subunit (eg the ε-chain)
– armored: pairs a CAR with an additional protein, such as a cytokine
enhance the immune response
– chimeric autoantibody receptor
uses an autoantigen as its binding domain
CAR-T landscape in autoimmune disease:
– several diseases
systemic lupus erythematosus (most studied), systemic sclerosis, idiopathic inflammatory myopathy, myasthenia gravis, multiple sclerosis
– common target CD19, but also BCMA
– products
Let’s dig into the results next.
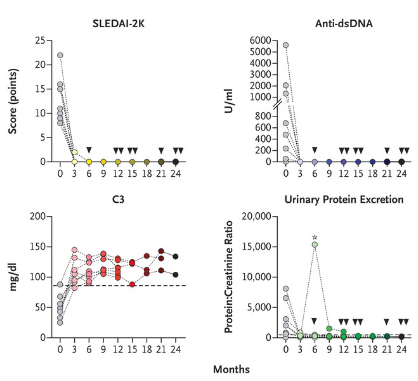
Lupus:
– anti-CD19 CAR T with remarkable results in severe, treatment-resistant lupus
achieved rapid remission after infusion
pathogenic autoantibodies dropped to normal levels
no serious side effects like cytokine release syndrome or neurotoxicity
– case series confirmed
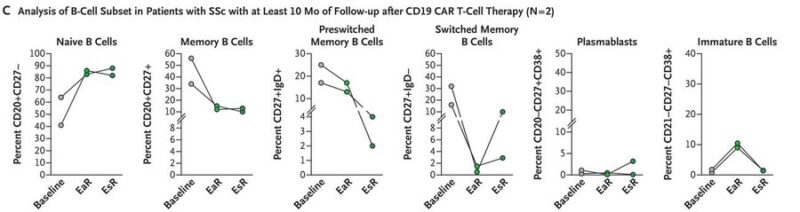
Systemic sclerosis:
– marked by skin thickening and organ fibrosis
– immune dysfunction
B cells promote inflammation + fibrosis
cytokine release + autoantibody production
– CAR-T induced symptom relief and disease stabilization
deep depletion of pathogenic B cells
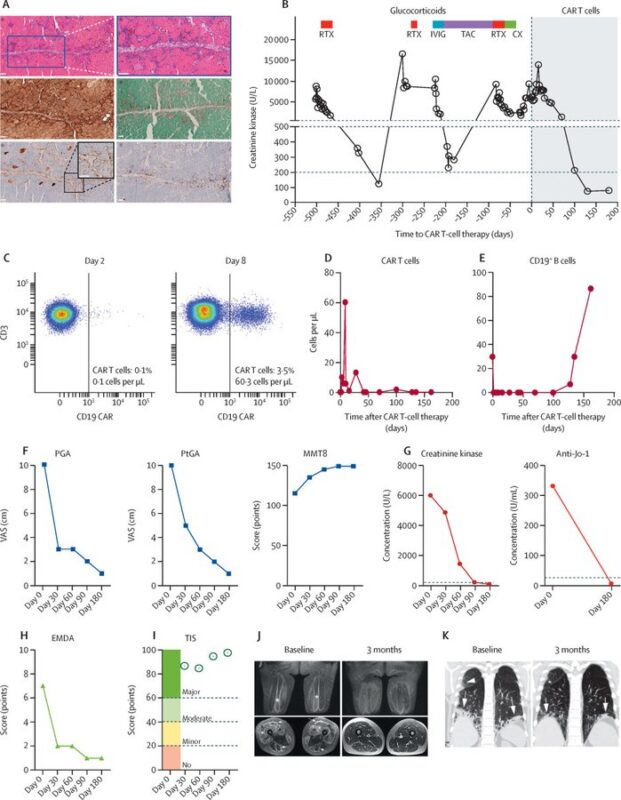
Anti-synthetase syndrome:
– rare condition characterized by inflammation in muscles, lungs, joints
– anti-CD19 improved muscle strength and endurance significantly
– Anti-Jo-1 antibodies dropped to undetectable levels, and MRI scans revealed resolution of muscle inflammation
Myasthenia gravis:
– neuromuscular disorder caused by autoantibodies attacking the neuromuscular junction
– there are both anti-CD19 and anti-BCMA reports
– no cytokine release syndrome or neurotoxicity
– clinically meaningful decreases on myasthenia gravis severity scales
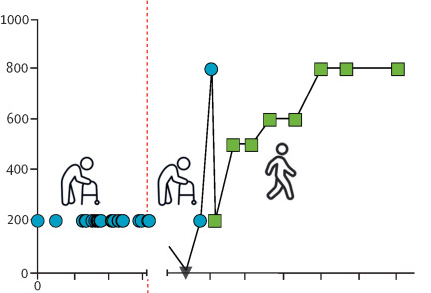
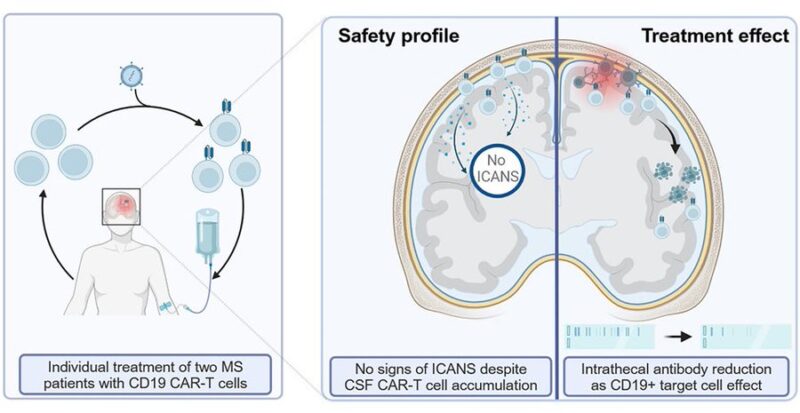
Multiple scerosis:
– first cases in progressive form with anti-CD19 CAR-T
expanded effectively in CNS
no neurotoxicity
– in 1 patient, intrathecal antibody levels in the CSF significantly decreased
– suggest potential in multiple sclerosis, but more evidence needed
Open issues:
– most patients achieve remission
BUT
not all auto-antibodies (anti-nucleosomes, Ro60) are reduced
disease may be driven by other B cell functions?
local production of cytokines?
pathogenic autoantibodies produced by CD19-negative long lived plasma cells?
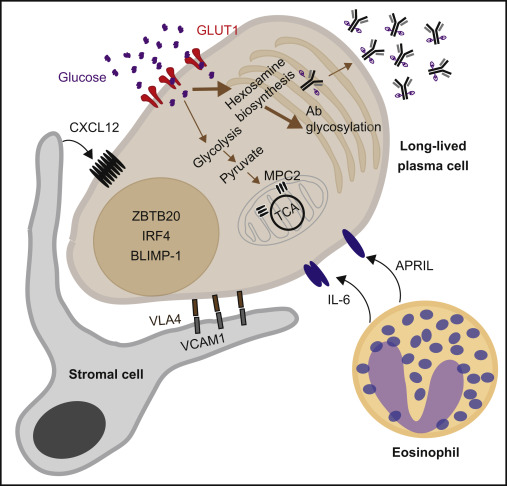
Long lived plasma cells:
– CD19-negative, which may also produce pathogenic autoantibodies
resistant to anti-CD19 therapies
– rely on proteasome to manage misfolded proteins and prevent cell stress
– alternative approaches: CD38-targeting (eg daratumumab or bortezomib)
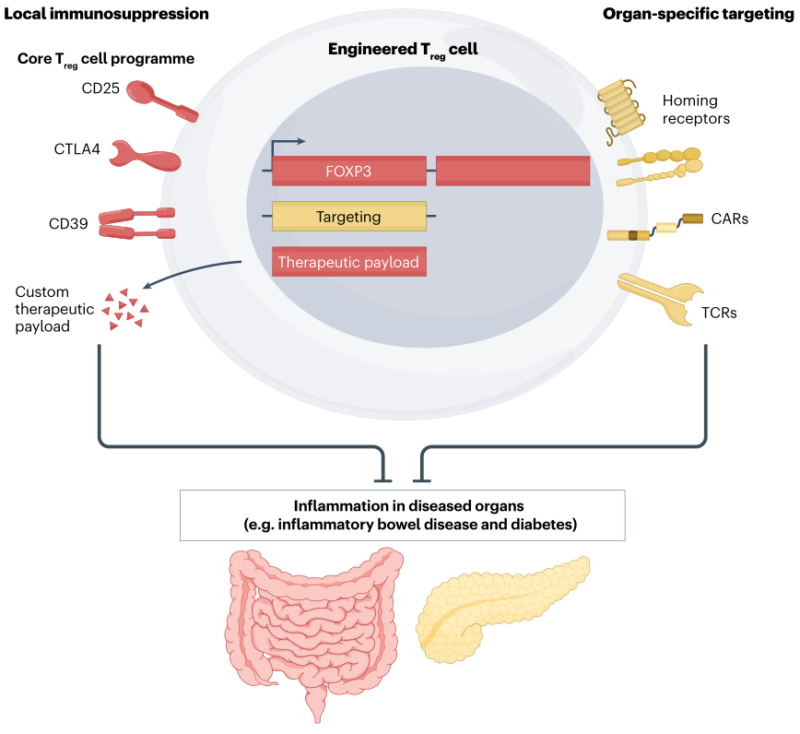
Role of CAR T regulatory cells?
– Tregs help maintain immune balance by reducing inflammation and autoimmunity
direct inhibitory interactions
release anti-inflammatory cytokines (IL-10,TGF-β) – expanded polyclonal Tregs have shown safety in autoimmune and alloimmune settings
– antigen-specific Tregs offer more targeted immune regulation
– advances in CAR technology now allow Tregs to be engineered with CARs, enhancing their ability to localize to sites of active inflammation
– eg, anti-CD19 CAR Tregs target B cells without depleting them entirely
– preclinical studies show reduced B cell activity and control diseases like graft-versus-host disease, with some evidence suggesting they may also reduce neurotoxicity in CAR T-treated patients
-promise for more precisely targeted, effective, safer treatments for autoimmune diseases
The dilemma of access:
– currently, there are ~40 clinical trials registered for CAR T therapy in autoimmune diseases
– a lot of enthusiasm
BUT
– autoimmune disease differs from malignancy
– narrow population could be eligible
– high cost
– unclear long-term infection risks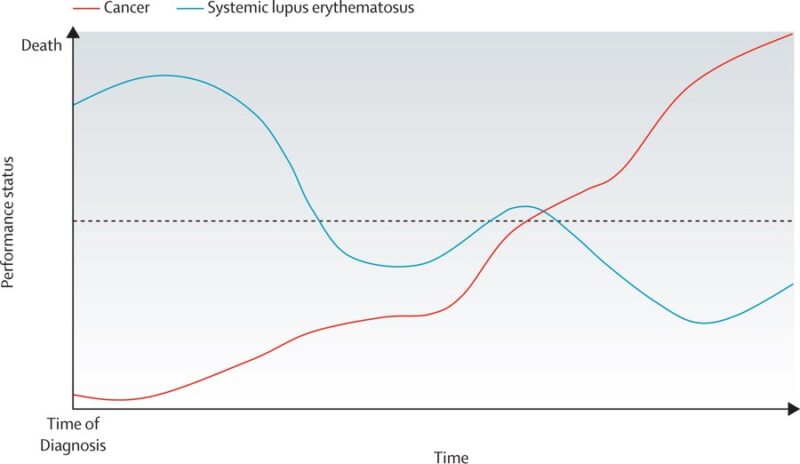
Bispecific antibodies as next game changer?
– Teclistamab, a CD3 and BCMA–bispecific antibody originally approved for multiple myeloma
– recent case in SLE showed reduced disease markers
– anti-dsDNA antibodies now undetectable
– rapid and extensive B and plasma cell depletion
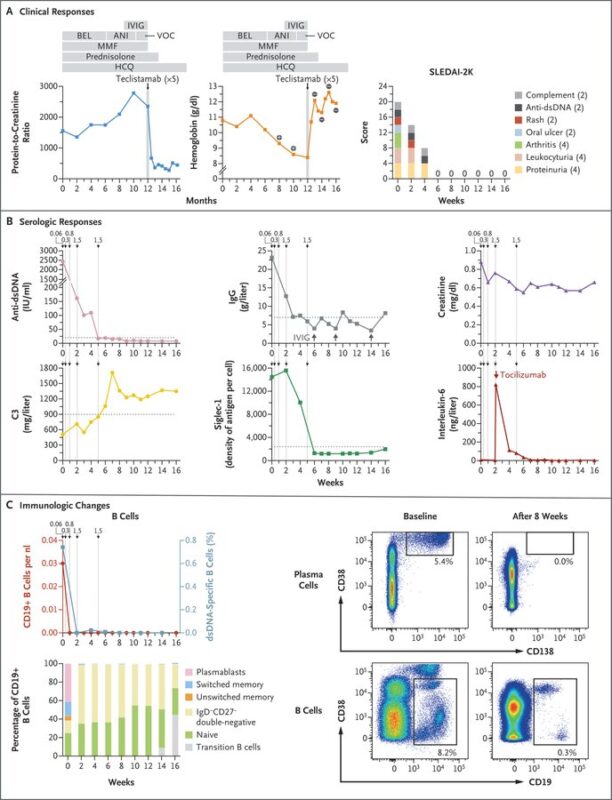
Summary of CAR T in autoimmune disease Targeting mostly CD19 and BCMA
- Deep remission potential
- Lupus mostly studied, but others are emerging
- Deplete circulating and tissue-resident B cells AND reduce autoantibodies and inflammation
- Very good safety profile “for now”
- CAR T regulatory cells and bispecific antibodies emerging alternatives
- Challenges: access, cost, still a lot of unknowns
- Thanks for reading and happy to receive comments and engagement. Follow for more content and make this place peaceful, loving and curious
More posts featuring Nico Gagelmann.
Nico Gagelmann is a physician and scientist who co-founded and co-chairs the European Society for Blood and Marrow Transplantation (EBMT) Trainee Committee, and he also serves as the chair of the EBMT subcommittee focused on CAR-T cell therapies for plasma cell disorders.
His work is particularly impactful in the realm of CAR-T treatments for multiple myeloma, where he has contributed to advancing research and clinical approaches.
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
