Revumenib in relapsed or refractory KMT2Ar acute leukemia – Syndax Pharmaceuticals
Nicole McNeer, Senior Medical Director and Clinical Leader at Syndax Pharmaceuticals, shared a post by Syndax Pharmaceuticals, adding:
”Another testament to our team’s dedication, innovation, collaboration, and speed. Proud to be clinical lead for the menin program through some amazing milestones, all in less than two years since joining Syndax – completion of the Phase 1 study, achievement of Breakthrough Therapy Designation, early completion of the Phase 2 study in KMT2Ar leukemia with positive results, and now acceptance into the Real-Time Oncology Review program! Let’s go team!”
Quoting Syndax Pharmaceuticals‘ post:
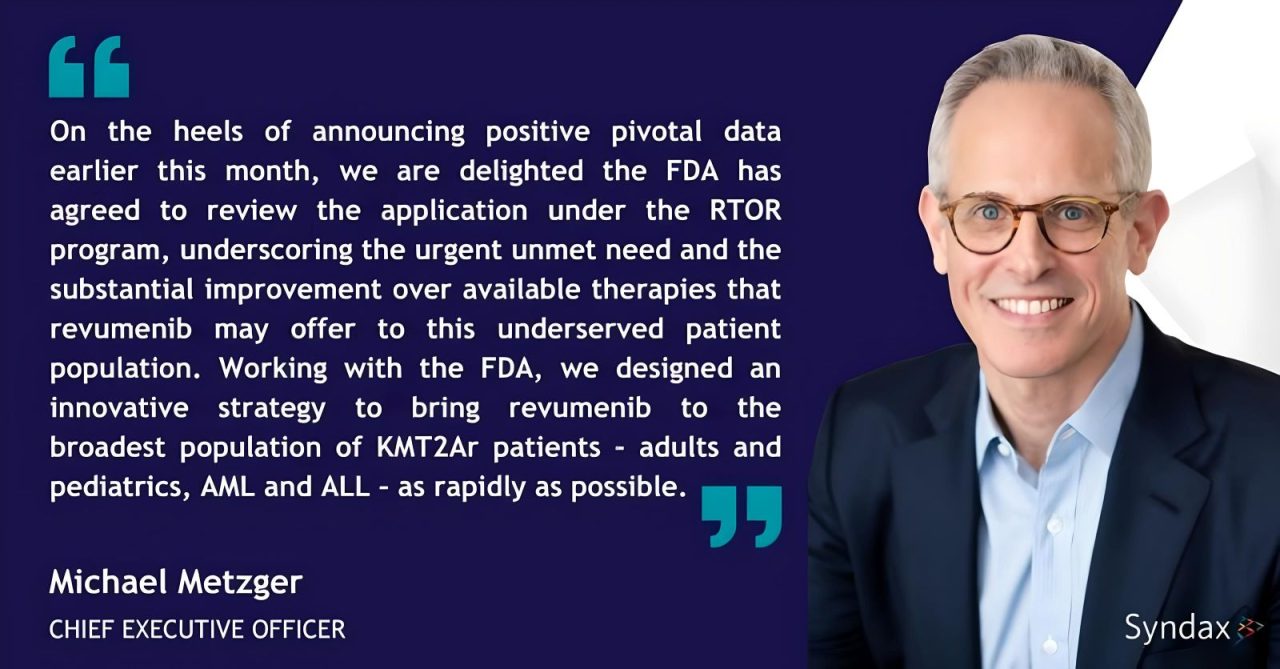
“We’re thrilled to announce that we will be submitting a New Drug Application (NDA) for revumenib in relapsed or refractory (R/R) KMT2Ar acute leukemia, including acute myeloid leukemia (AML) and acute lymphoid leukemia (ALL), under the U.S. FDA Real-Time Oncology Review (RTOR) program.
We look forward to continuing to work with the FDA to bring this potential treatment option to patients in need as rapidly as possible.”
For more details click here.
Source: Nicole McNeer/LinkedIn and Syndax Pharmaceuticals/LinkedIn
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
