
FDA approves Novocure’s treatment for mNSCLC
The FDA has granted approval for Novocure’s Optune Lua as a treatment option for metastatic non-small cell lung cancer (NSCLC).
On October 15, 2024, the U.S. Food and Drug Administration (FDA) granted approval for Optune Lua, a groundbreaking treatment for adult patients with metastatic non-small cell lung cancer (mNSCLC) who have experienced disease progression following platinum-based chemotherapy. This device can be used in conjunction with PD-1/PD-L1 inhibitors or docetaxel, marking it as the first of its kind for this patient demographic.
Key Highlights of Optune Lua
Mechanism of Action:
Optune Lua is a wearable device that delivers Tumor Treating Fields (TTFields), which are alternating electric fields that target and disrupt the division of cancer cells. This process leads to cell death while sparing healthy cells13.
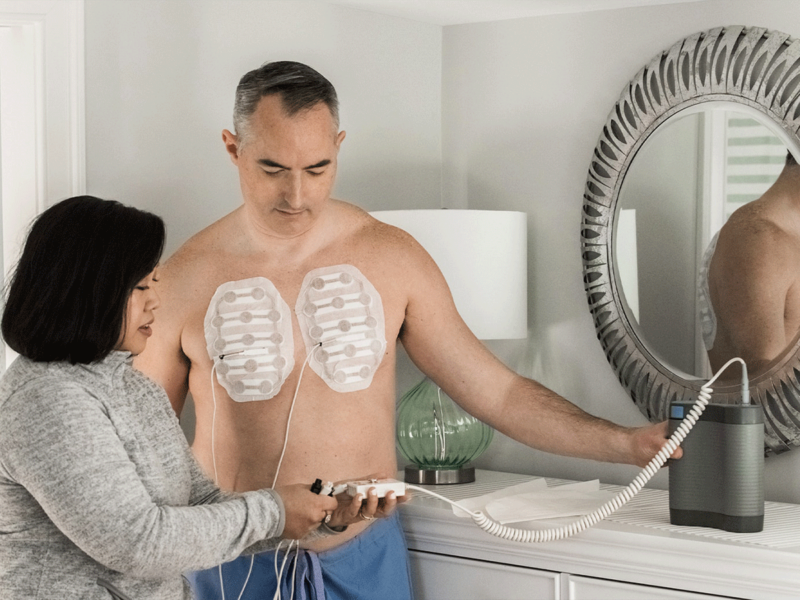
Clinical Evidence:
The pivotal Phase 3 LUNAR trial demonstrated a statistically significant improvement in median overall survival (OS) for patients using Optune Lua alongside standard treatments. Specifically, patients receiving Optune Lua with PD-1/PD-L1 inhibitors experienced a median OS of 19.0 months, compared to 10.8 months for those on PD-1/PD-L1 inhibitors alone (P=0.02).
Additionally, those using Optune Lua with docetaxel had a median OS of 11.1 months, compared to 8.9 months for docetaxel alone, although this difference did not reach statistical significance16.
Safety Profile:
The device-related adverse events primarily involved skin issues under the transducer arrays, occurring in 63.1% of patients. Most were low-grade, with only 4% experiencing more severe skin toxicity requiring treatment interruption67.
Significance of Approval
This approval represents a notable advancement in treatment options for mNSCLC, particularly as it provides a new avenue for patients who have limited effective therapies available after failing platinum-based chemotherapy. Asaf Danziger, CEO of Novocure, emphasized the company’s commitment to improving survival rates in challenging cancers and expressed gratitude towards the community involved in the clinical trials16.

Dr. Ticiana Leal, a primary investigator in the LUNAR study, highlighted that this is the first substantial improvement in overall survival for mNSCLC patients in over eight years, making Optune Lua a compelling option for both patients and healthcare providers seeking better treatment alternatives.

Possible side effects of Optune Lua
The most common side effects associated with Optune Lua when used in combination with certain immunotherapy and chemotherapy drugs include:
- Dermatitis
- Muscle, bone, or joint pain
- Fatigue
- Anemia
- Alopecia
- Dyspnea
- Nausea
- Cough
- Diarrhea
- Anorexia
- Pruritus
- Leukopenia
- Pneumonia
- Respiratory tract infections
- Localized edema
- Rash
- Pain
- Constipation
- Skin ulcers
- Hypokalemia
- Hypoalbuminemia
- Hyponatremia
- Dysphagia
In addition to these common side effects, other potential adverse effects related to the use of Optune Lua may include:
- Skin irritation from treatment
- Allergic reactions to the adhesive or gel
- Overheating of the array, leading to pain or local skin burns
- Infections at the contact sites of the arrays
- Local warmth and tingling sensations beneath the arrays
- Muscle twitching
- Skin breakdown or ulcers.
Healthcare professionals and organizations shared about this on social media:
“FDA approves Novocure’s treatment for metastatic non-small cell lung cancer (mNSCLC) for use concurrently with PD-1/PD-L1 inhibitors or docetaxel in adult patients with mNSCLC who progressed on or after a platinum-based regimen.”

“Congrats Novocure!
Future indication for pancreatic cancer??
Ph3 PANOVA3 trial readout is pending for LAPC.
Ph2 trial of ablative SMART and TTFields for LAPC is open…TTFields intended to delay or prevent liver/peritoneal progression…. please share!”
“Its only Tuesday and we have another FDA Oncology approval for Novocure’s Optune Lua TTF (device) plus docetaxel or Immunotherapy for 2L NSCLC:
- Ph3 LUNAR study
- OS in ImmunoTx and TTF: 19m vs 10.8m.
- Pt compliance with a vest for 18 hrs/day.”
About Novocure
Novocure is a global oncology company focused on extending survival for patients with aggressive cancers through its innovative therapy known as Tumor Treating Fields (TTFields). The company has successfully commercialized products approved in various countries for treating adult patients with conditions such as glioblastoma and malignant pleural mesothelioma.
Additionally, Novocure is actively conducting clinical trials exploring the application of TTFields in several cancers, including brain metastases, gastric cancer, liver cancer, non-small cell lung cancer (NSCLC), pancreatic cancer, and ovarian cancer.
Company Overview
- Headquarters: Root, Switzerland
- Regional Centers: Portsmouth, New Hampshire; Tokyo, Japan
- Research Center: Haifa, Israel
Products and Clinical Research
Novocure’s products are designed to provide new treatment options for patients facing limited alternatives. The recent approval of Optune Lua for use in metastatic NSCLC marks a significant advancement in treatment options for patients who have progressed after platinum-based chemotherapy. This device can be used concurrently with PD-1/PD-L1 inhibitors or docetaxel.

-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
