Monica Bertagnolli: Repurposed Cancer Drug Shows Effectiveness in Treating Rare Blood Vessel Disorder
Monica Bertagnolli recently shared a post about repurposed cancer drug effects in treating rare blood vessel disorders. Read her post on LinkedIn below:
“Developing a new drug from scratch can take a decade or more. But sometimes promising treatment options come from repurposing existing drugs for completely different medical conditions.I’m happy to share a new example of this: a cancer drug called pomalidomide that was found in a clinical trial to be safe and effective for treating a blood disorder called hereditary hemorrhagic telangiectasia (HHT).
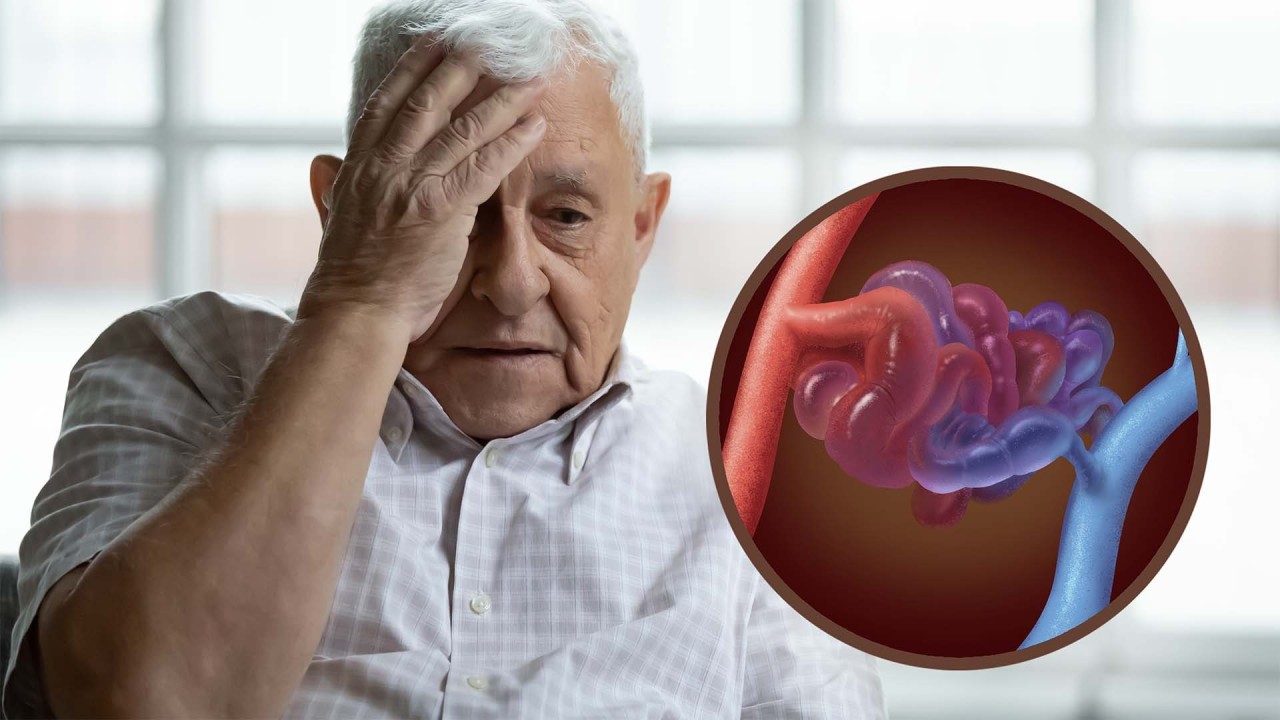
HHT is an inherited blood vessel disorder that can cause excessive or even life-threatening bleeding. The disease is rare, affecting about 1 in every 5,000 people worldwide, but because HHT is poorly understood and often misdiagnosed, its true incidence is likely greater.
Most people with HHT experience recurrent severe nosebleeds, often in combination with mental health disorders such as depression and post-traumatic stress disorder, as well as other health conditions. HHT can also worsen with age and impact quality of life.
However, recent findings from an NIH-supported clinical trial, reported in the New England Journal of Medicine, show that daily treatment with pomalidomide in people with HHT led to a significant reduction in nosebleed severity.
Compared to trial participants taking a placebo, those taking pomalidomide needed fewer blood or iron transfusions and reported improvements in their quality of life. Because of these results, the trial was stopped months ahead of schedule, having found sufficient evidence that the treatment was safe and effective.
In people with HHT, blood vessels become unusually tangled and twisted, causing them to be fragile and prone to bleeding. This leads to excessive nosebleeds and, in some cases, gastrointestinal or other internal bleeding that can cause serious complications in the lungs, liver, and brain.
Existing options for treating HHT include procedures to seal off malformed vessels in the nose and GI tract and drugs prescribed off-label to help reduce bleeding by stabilizing blood clots. But HHT symptoms tend to get worse over time, and there haven’t been any Food and Drug Administration-approved treatments for controlling this disease long-term.
The new findings come from a Cleveland Clinic team led by Keith McCrae. McCrae’s inspiration arose from a patient with HHT he’d treated many years ago, who was having nosebleeds and severe GI bleeding.
In search of alternatives to an invasive and life-altering surgical procedure, he looked for other options and discovered there had been some indications that thalidomide, a drug approved to treat multiple myeloma, might also have benefits for treating HHT.
McCrae tried thalidomide with this patient and a few others who showed similar HHT symptoms and found it to be effective. But he knew that treatment with thalidomide can have serious side effects. McCrae wanted to find an alternative for HHT with fewer safety concerns.
He found it in pomalidomide, which is FDA-approved for treating cancers of the bone marrow. A pilot study he conducted in people with HHT suggested that the drug was indeed promising. But he needed to put it to the test in a larger clinical trial.
In the trial, his team enrolled 144 adults with HHT at 11 U.S. medical centers. All the study’s participants had moderate to severe nosebleeds requiring transfusions of iron or blood. They were randomly assigned to receive either a pomalidomide pill or a sugar pill placebo daily over a period of 24 weeks.
About five months before the trial was scheduled to close, a planned interim analysis showed that the participants who took pomalidomide had nosebleeds that were less severe, leading to fewer transfusions and improvement in their reported quality of life. Because the findings met a prespecified threshold for efficacy, the trial was closed to enrollment early.
The researchers suggest pomalidomide works by limiting the growth of abnormal blood vessels. It may also make blood vessels less fragile and prone to leaks. But there is much more to learn about the mechanisms behind the disease. More study is needed to understand how the treatment might work longer-term and in those with more severe symptoms.
The drug also comes with risk for adverse effects including low white blood cell count, constipation, and rash. But this trial demonstrating the safety and efficacy of a possible new therapeutic option for HHT is encouraging, and the findings are a step forward in improving the lives of people living with this condition.”
More posts featuring Monica Bertagnolli on Oncodaily.com.
Monica Bertagnolli is the Director of the National Institute of Health (NIH), USA. She is the President of Alliance for Clinical Trials In Oncology Foundation.
She previously served as the Richard E. Wilson Professor of Surgery in the field of surgical oncology at Harvard Medical School, a surgeon at Brigham and Women’s Hospital, and a member of the Gastrointestinal Cancer Treatment and Sarcoma Centers at Dana-Farber Cancer Institute.
Dr. Bertagnolli is the Founding Chair of the minimal Common Oncology Data Elements (mCODE) executive committee and has held multiple positions nationally, including being Past President and Chair of the Board of Directors of the American Society of Clinical Oncology. In 2021, she was elected to the National Academy of Medicine, having previously served on the National Academies National Cancer Policy Forum.
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
