Quoting Peter Westcott, Assistant Professor at the Cold Spring Harbor Lab, on X/Twitter:
“Out today, with Jacks Lab, Isidro Cortes-Ciriano, Francesc Muyas, we used new mouse models of spontaneous lung and colon cancer to delineate a mechanism for why many patients with DNA mismatch repair deficient (MMRd) tumors may not respond to immunotherapy.
I couldn’t be more pleased with how this work came together and its home at Nature Genetics. I am incredibly grateful to our colleagues, Massachusetts Institute of Technology (MIT), Cold Spring Harbor Lab, European Molecular Biology Laboratory-European Bioinformatics Institute and to Safia Danovi for her skilled handling of our manuscript and the peer review process, and to our reviewers.
See our summary from the pre-print, which covers more details of the punchline: intratumoral heterogeneity of mutations is bad for the immune response in MMRd cancer! Key experiments deepening mechanism were also added in the peer review process.
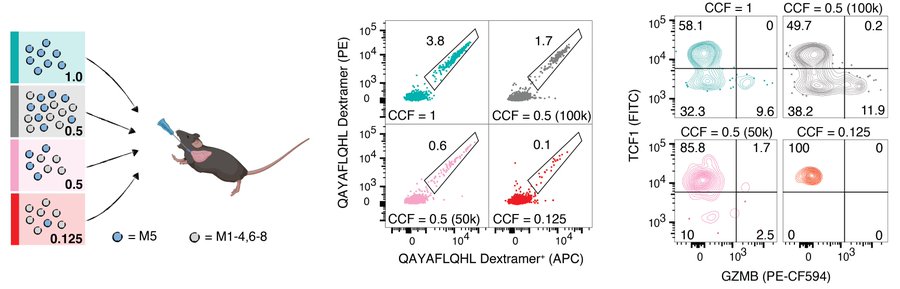
We identified bona fide neoepitopes presented on MMRd tumors in the models and showed that in clonal mixing experiments, the neoantigen-specific CD8 T-cell response decreases in magnitude and effector differentiation as clonal fraction decreases.
We also reanalyzed human clinical trial data of colorectal and gastric cancers with MMRd and show that clonal, but not subclonal, neoantigen burden is predictive of response to immune checkpoint blockade (anti-PD-1).
These results should give serious pause to clinical efforts that purposefully mutagenize tumors with the intent of making them more immunogenic. Such efforts are likely to fail, exacerbating intratumoral heterogeneity and potentially accelerating malignant evolution.
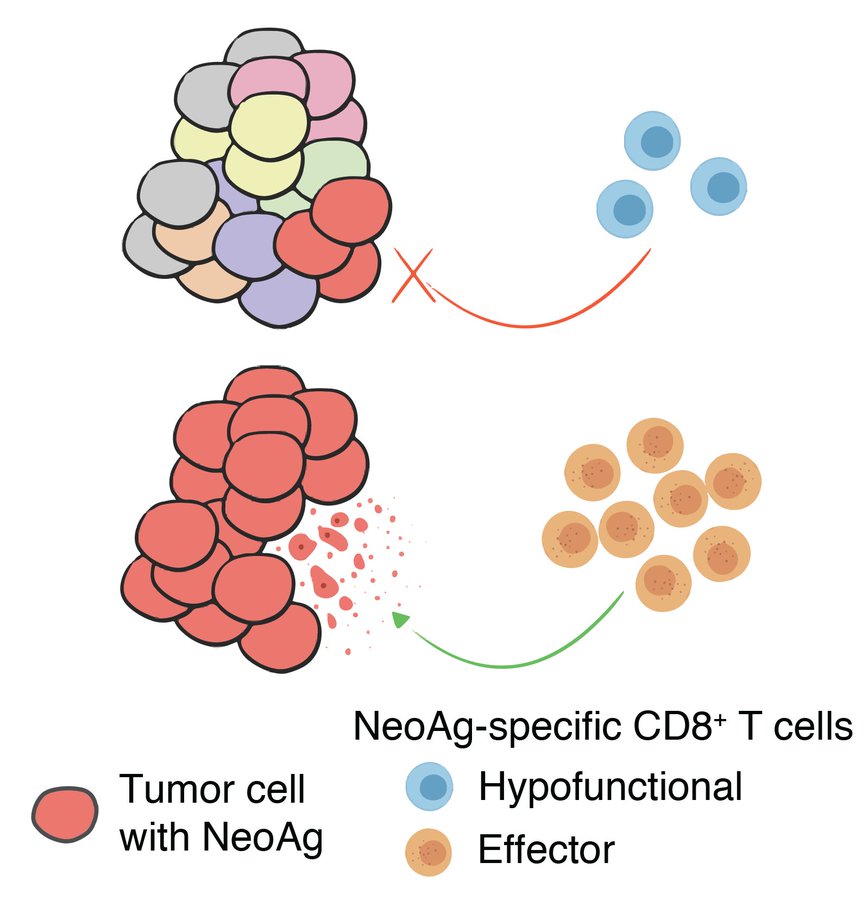
I also want to highlight a result I personally find fascinating: we sequenced MMRd tumors that arose spontaneously in mice with and without T cells (anti-CD4/CD8) and saw no difference in overall neoantigen burden, but a stark difference in neoantigen clonal architecture.
This completely revises the way I think about “immunoediting“, à la Drs. Old and Schreiber. Immune surveillance eliminates tumor clones with strong neoantigens, but only if they are sufficiently clonal. That means that potent neoantigens likely lurk in the subclonal fraction.
Indeed, we showed this in our retransplant survival experiments. Single-cell clones from an otherwise nonimmunogenic MMRd tumor (but not MMR proficient tumor) were highly immunogenic when retransplanted clonally. Very interesting questions arise from this observation.
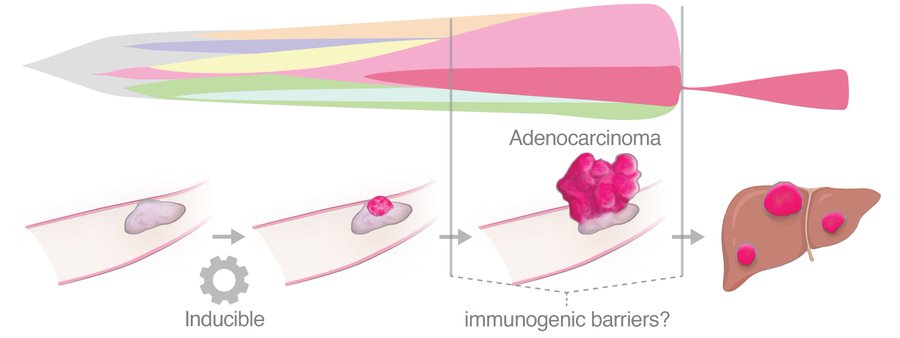
Here’s one: How do tumors BECOME immunogenic? Cancer progression is often stepwise, punctuated by distinct selective events that undoubtedly reshape the clonal architecture of neoantigens. Are formerly subclonal neoantigens revealed to the immune system during progression?
My group, Cold Spring Harbor Lab, is passionate about these questions and we are developing new mouse models to dissect tumor-immune crosstalk during clonal evolution. We are hiring postdocs and technicians, please reach out for more info. Job postings coming soon!”
For the article click here.
For details here.
Source: Peter Westcott/Twitter