Francisco J. Esteva, Professor of Medicine at the Zucker School of Medicine, shared an article by Fabio Conforti, et al. on LinkedIn:
“What should be the primary endpoint for FDA registration trials in early-stage breast cancer?
Overall survival – OS? Invasive Disease-Free Survival – iDFS? Pathological complete response -pCR?
Overall survival would be ideal, but it is not considered necessary because of the time it would take to approve novel treatments for patients who need them.
A recent metanalysis of randomized clinical trials showed that iDFS is a stronger surrogate for OS than pCR, especially in premenopausal patients and those with low-grade or lobular tumors.
However, it is reassuring to read that pCR remains valuable for guiding personalized adjuvant therapy, especially in HER2-positive and triple-negative cancers. In this study, as shown previously, patients who achieved pCR had longer OS than patients with residual invasive cancer, regardless of the treatment arm they were assigned to.
Therefore, while pivotal studies may focus on iDFS to approve new drugs, as a medical oncologist I will continue to rely on pCR to guide personalized treatment on my patients.”
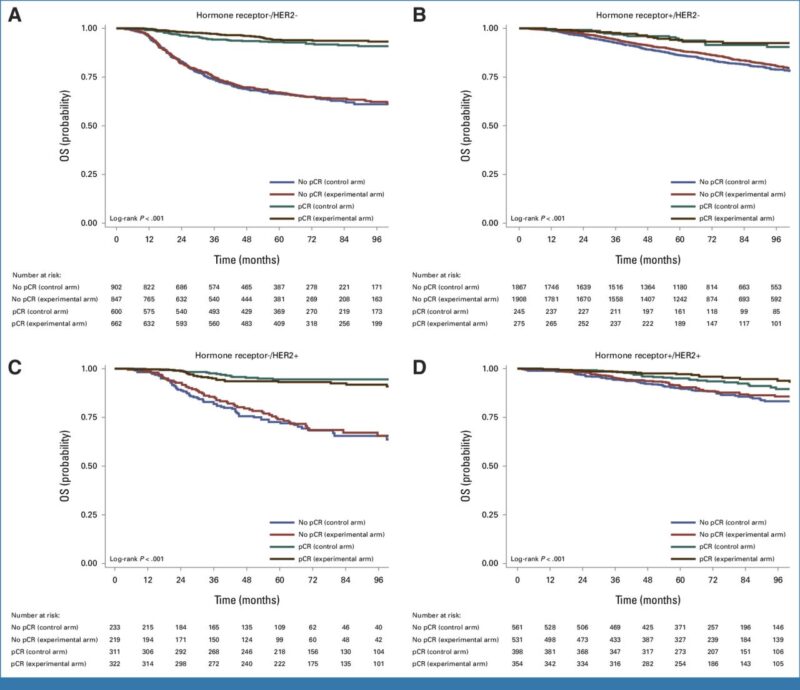
Surrogate End Points for Overall Survival in Neoadjuvant Randomized Clinical Trials for Early Breast Cancer.
Authors: Fabio Conforti, et al.



