PLATO-ACT 4 Trial presented at ESTRO 2025 by Prof. David Sebag-Montefiore, marks a pivotal advancement in the treatment of early-stage anal squamous cell carcinoma. This phase 2 randomized controlled trial evaluated whether dose-reduced chemoradiotherapy (dr-IMRT) could maintain oncological outcomes while improving tolerability compared to standard-dose IMRT (sd-IMRT). With over 160 patients enrolled and a 3-year locoregional failure-free rate of 87.6% in the reduced-dose arm, the PLATO-ACT4 trial provides strong evidence supporting de-escalation of radiotherapy for select patients with T1–T2N0 anal cancer.
Rationale and Background
Anal cancer is rare, with fewer than 4,000 global cases annually, but incidence is projected to rise significantly by 2045.
While standard chemoradiotherapy offers high cure rates, acute and long-term toxicity remain a major burden, and the optimal radiation dose for early-stage disease has not been well defined for over 25 years.
The PLATO-ACT4 trial was designed to evaluate whether de-escalating radiotherapy dose could maintain efficacy while improving tolerability. 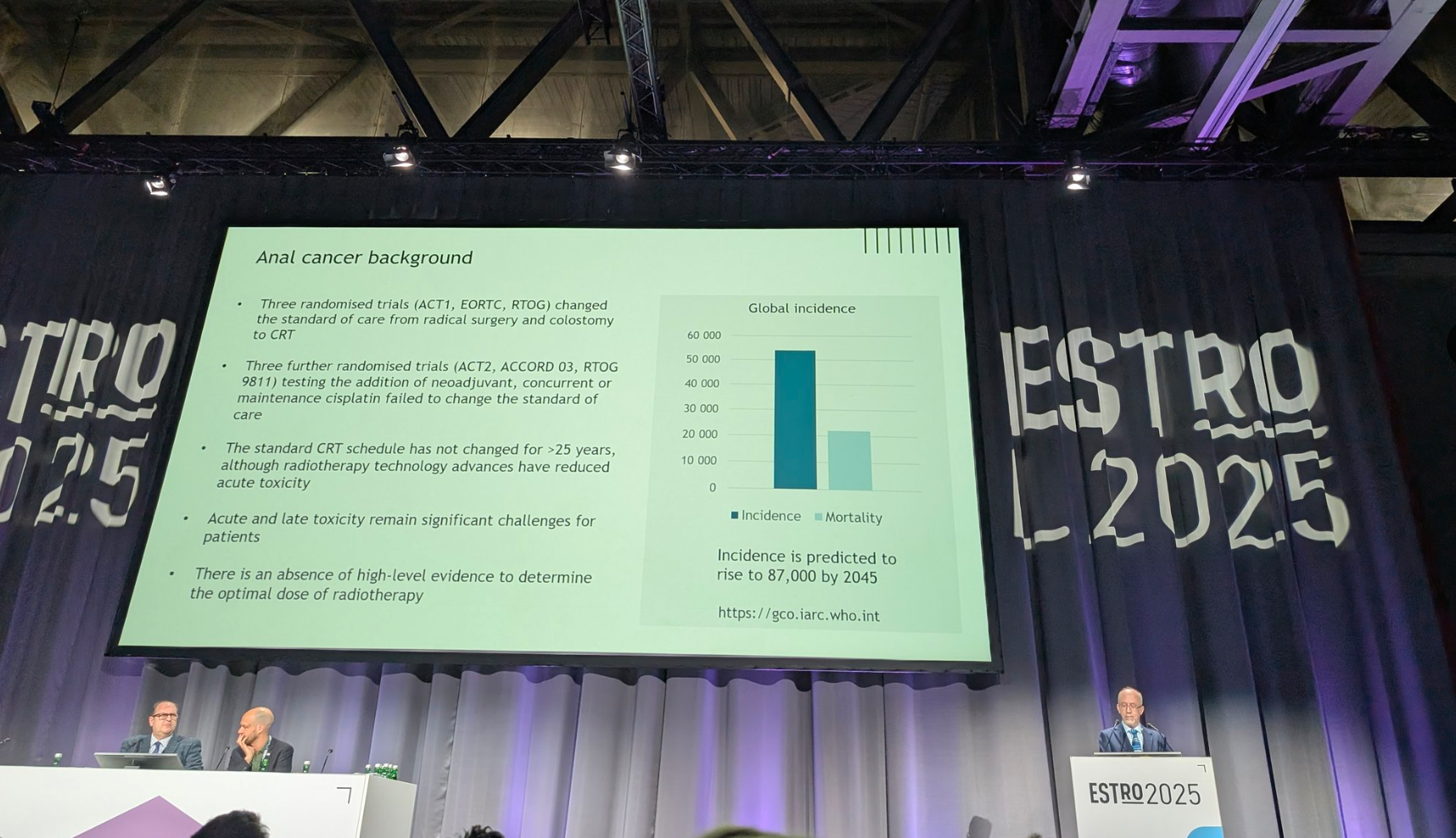
PLATO-ACT 4 Study Design
- Phase II, multicenter randomized controlled trial
- 2:1 randomization: dr-IMRT vs. sd-IMRT
- Total sample size: 162 (including subgroup analysis by p16 status)
Treatment details
- All patients received:
- Mitomycin-C (12 mg/m², Day 1)
- Capecitabine (825 mg/m² twice daily on radiotherapy days)
- dr-IMRT: 41.4 Gy in 23 fractions (nodal dose: 34.5 Gy)
- sd-IMRT: 50.4 Gy in 28 fractions (nodal dose: 40 Gy)
Primary endpoint: 3-year locoregional failure-free rate (LRFFR)
Results
A total of 160 patients were enrolled in the study, with a median age of approximately 66 years. Notably, over 90% of participants were p16-positive.
At the 3-year mark, the loco-regional failure-free rate (LRFFR) was 87.6% in the de-escalated radiation intensity-modulated radiotherapy (dr-IMRT) arm, compared to 83.6% in the standard-dose IMRT (sd-IMRT) arm. The primary endpoint was achieved, as the lower bound of the 90% confidence interval in the dr-IMRT group remained above the 80% threshold. Importantly, no isolated nodal failures were observed in either treatment arm. Disease-free survival (DFS) at 3 years was 84.5% for dr-IMRT and 83.4% for sd-IMRT. 3-year overall survival was
- dr-IMRT: 98.1%
- sd-IMRT: 92.6%
Patient-Reported Outcomes (PROs)
- Global quality of life and bowel function declined during treatment but returned to baseline by 6 months in both arms
- Sexual function recovered in dr-IMRT group by 6 months, but remained impaired in sd-IMRT arm
- This is the first RCT to report ANL27 data, providing anal cancer-specific quality of life metrics
Key Takeaways
- Reduced dose IMRT with concurrent MMC and capecitabine resulted in:
- 87.6% locoregional failure-free rate at 3 years – primary endpoint met
- 84.5% disease-free survival at 3 years
- 98.1% overall survival at 3 years
- No isolated nodal failures
- Patient-reported outcomes measuring late toxicity were similar
- The shorter, reduced-dose IMRT regimen offers major advantages to patients, their carers, and healthcare systems
→ It should be considered a new treatment option for patients with early-stage anal cancer
Read Simultaneous Publication on Lancet Oncology entitled Standard versus reduced-dose chemoradiotherapy in anal cancer (PLATO-ACT4): short-term results of a phase 2 randomised controlled trial.

About ESTRO 2025
ESTRO 2025 brings together around 7,000 participants from over 80 countries, showcasing the latest research in clinical radiation oncology, radiobiology, medical physics, technology, and brachytherapy. Leading doctors and scientists from around the world present groundbreaking findings, in line with the conference theme: “Transformative innovation through collaboration”. ESTRO 2025 is the annual congress of the European Society for Radiotherapy and Oncology (ESTRO), an organisation dedicated to advancing cancer treatment through radiotherapy and multimodal approaches. ESTRO promotes education, science, and research and advocates for universal access to radiotherapy. With nearly 10,000 members worldwide, it supports radiation oncology professionals and the broader oncology community in their daily practice.
Read ESTRO 2025 Updates on OncoDaily