
Sergio Cifuentes Canaval: Pembrolizumab’s Role in Hepatocellular Carcinoma
Sergio Cifuentes Canaval, Cancer Research Project Manager at CENEIT México, shared a post on LinkedIn:
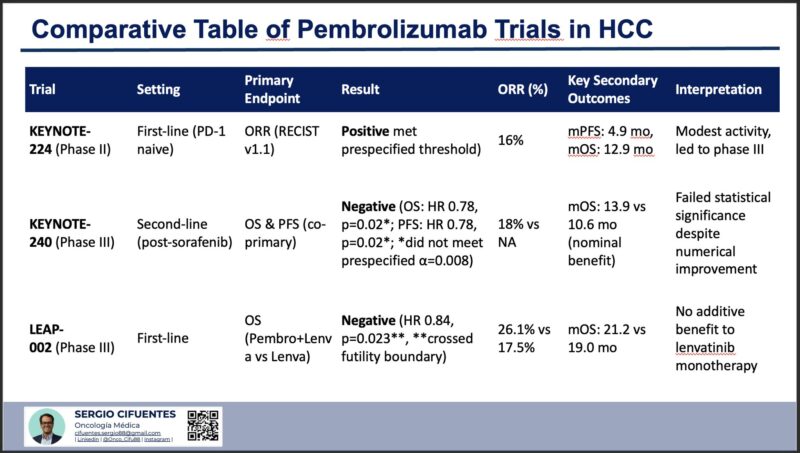
“Why Pembrolizumab Failed in HCC?
Recent discussions have questioned pembrolizumab’s role in hepatocellular carcinoma (HCC) after disappointing trial results. Let’s break down the evidence:
Key Trials and Outcomes
1. KEYNOTE-224 (Phase II):
– 1st-line
– Primary Endpoint: ORR (RECIST v1.1)
– Result: Positive (16% ORR)
– ORR: 16%
– Takeaway: Modest activity, but insufficient for a standalone therapy.
2. KEYNOTE-240 (Phase III):
– 2nd-line
– Primary Endpoint: OS and PFS
– Result: Negative, (OS: HR 0.78, *p=0.02*)
– ORR: 18%
– Takeaway: Nominal OS benefit, but missed statistical significance.
3. LEAP-002 (Phase III):
– 1st-line
– Primary Endpoint: OS (Pembro + Lenvatinib vs. Lenvatinib)
– Result: Negative, (HR 0.84, *p=0.023*)
– ORR: 26.1% vs. 17.5%
– Takeaway: No survival benefit despite higher ORR.
Why Did Pembrolizumab Fail in HCC?
– Modest Efficacy: ORRs (16–26%) lag behind current standards (e.g., atezolizumab + bevacizumab: 27–30%).
– Statistical Misses: Phase III trials showed numerical OS improvements but failed stringent significance thresholds.
– Benchmark Problem: Competing regimens (IMbrave150, HIMALAYA) demonstrated clearer survival benefits, raising the efficacy bar.
Key Lessons for Drug Development
1. Metastatic failure predicts adjuvant outcomes: If a drug underperforms in advanced disease, testing it in earlier settings may not be justified.
2. Combination ≠ Success: LEAP-002 proved that adding PD-1 inhibition to lenvatinib doesn’t guarantee improved survival.
3. Biomarker refinement needed: PD-L1/TMB haven’t reliably predicted response in HCC—future trials should explore better patient selection.
While pembrolizumab reshaped oncology in other cancers, its HCC journey highlights that not all immunotherapies are equal. Moving forward, prioritizing mechanisms with stronger biological rationale (e.g., dual checkpoint inhibition in HIMALAYA) may yield better outcomes.
What’s your take? Should pharma abandon pembrolizumab in HCC, or is there still a niche role?
*(Data sourced from KEYNOTE-224, KEYNOTE-240, LEAP-002, IMbrave150, and HIMALAYA trials.)”
More posts featuring Sergio Cifuentes Canaval.
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
