NIVOPOSTOP (GORTEC 2018-01): A Phase III Trial of Adjuvant Nivolumab in High-Risk Resected Head and Neck Squamous Cell Carcinoma
Nivolumab have demonstrated substantial clinical activity in recurrent/metastatic HNSCC. This led to the hypothesis that their earlier use in the adjuvant setting might improve outcomes by eliminating micrometastatic disease and modulating the postoperative immune environment. The NIVOPOSTOP (GORTEC 2018-01) study was designed to test this hypothesis by evaluating adjuvant nivolumab following postoperative CRT in patients with high-risk HNSCC.
Trial Design and Objectives
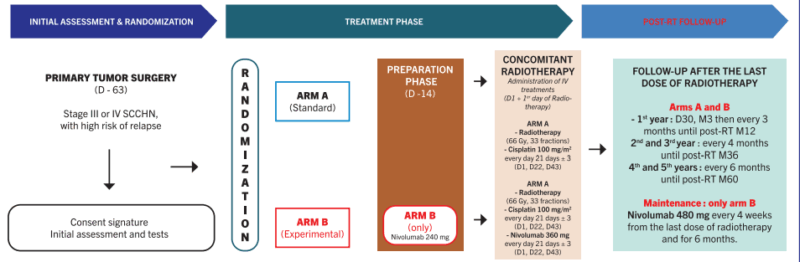
NIVOPOSTOP (GORTEC 2018-01) is a Phase III, randomized, open-label, multicenter trial designed to assess the efficacy of adjuvant nivolumab following standard postoperative chemoradiotherapy (CRT) in patients with resected head and neck squamous cell carcinoma (HNSCC) at high risk of recurrence. The study was coordinated by the Groupe Oncologie Radiothérapie Tête et Cou (GORTEC) and led by Prof. Jean Bourhis (CHUV, Lausanne).
Patients eligible for this trial had high-risk pathologic features—specifically positive surgical margins or extranodal extension (ENE)—following curative resection of stage III–IVa HNSCC. These features are known to confer significantly worse prognosis, even after CRT (Cooper et al., 2004). Standard CRT improves locoregional control but fails to significantly reduce distant metastases in this population (Bernier et al., 2005), suggesting a role for systemic adjuvant therapy.
The study enrolled patients who had completed definitive surgery followed by CRT (66–70 Gy with concurrent cisplatin 100 mg/m² every 3 weeks). After completing CRT and confirming absence of residual disease, patients were randomized in a 1:1 ratio to one of two arms:
• Experimental Arm (Nivolumab): Patients received nivolumab 240 mg IV every 2 weeks for 16 weeks, followed by 480 mg every 4 weeks, for a total of up to 12 months of immunotherapy.
• Control Arm (Observation): Patients underwent standard surveillance without further systemic treatment, in line with existing clinical practice.
The primary endpoint of the trial was disease-free survival (DFS), defined as the time from randomization to the first evidence of locoregional recurrence, distant metastasis, or death from any cause. Secondary endpoints included overall survival (OS), distant metastasis-free survival (DMFS), locoregional control (LRC), treatment-related toxicity, and quality of life outcomes.
The trial was statistically powered to detect a hazard ratio (HR) of 0.70 in favor of nivolumab, corresponding to an approximate 10–12% absolute improvement in 2-year DFS. The study included pre-planned interim analyses and subgroup evaluations based on tumor site, HPV status (only HPV-negative oropharyngeal cancers were eligible), and smoking history.
The trial design was conceptually informed by prior success of adjuvant PD-1 blockade in other solid tumors such as non-small cell lung cancer (Forde et al., 2022) and melanoma (Weber et al., 2017), as well as immunologic data suggesting CRT-induced antigen release and increased PD-L1 expression may create an optimal setting for immune checkpoint inhibition (Sharabi et al., 2015).
Patient Population
Eligible patients had histologically confirmed squamous cell carcinoma of the oral cavity, oropharynx (excluding HPV-positive), hypopharynx, or larynx. Inclusion criteria required high-risk features such as ENE or positive surgical margins. Patients had to have completed adjuvant CRT with no evidence of residual disease on imaging or clinical assessment.
Results and Safety
At the time of the primary analysis, with a median follow-up of 26 months, the NIVOPOSTOP (GORTEC 2018-01) trial met its primary endpoint, demonstrating a statistically significant improvement in disease-free survival (DFS) for patients who received adjuvant nivolumab compared to those under observation alone.
Patients in the nivolumab arm achieved a 2-year DFS of 67.4%, compared to 56.6% in the observation group. This corresponded to a hazard ratio (HR) of 0.70 (95% CI: 0.52–0.94), indicating a 30% relative reduction in the risk of recurrence or death (Bourhis et al., 2025). The p-value of 0.018 confirmed the result was statistically significant. Notably, benefit from adjuvant nivolumab was consistent across subgroups defined by tumor location, margin status, ENE presence, and smoking history.
Distant metastasis-free survival (DMFS) also favored the nivolumab arm, suggesting that early systemic immune intervention may reduce the development of micrometastases, a major cause of mortality in high-risk HNSCC patients. While overall survival (OS) data were not yet mature at the time of reporting, early trends suggested improved survival curves in the experimental arm, pending longer follow-up.
Safety Profile
Adjuvant nivolumab was generally well tolerated, consistent with its established safety in other indications. The incidence of grade 3 or higher treatment-related adverse events (AEs) in the nivolumab group was approximately 7%, compared to less than 2% in the observation group (Bourhis et al., 2025). The most commonly reported immune-related adverse events (irAEs) included:
• Fatigue
• Rash
• Hypothyroidism
• Diarrhea
These were predominantly grade 1–2 in severity and manageable with standard supportive measures. No unexpected safety signals were observed, and there were no treatment-related deaths reported.
Importantly, the immunotherapy was administered in a population that had already undergone extensive treatment—including surgery and cisplatin-based CRT—yet tolerated further systemic therapy without major compromise to quality of life. These findings align with experiences in adjuvant immunotherapy across other tumor types (Weber et al., 2017; Forde et al., 2022), suggesting its feasibility and clinical value in the post-CRT setting for HNSCC.
Read More About Nivolumab on Oncodaily
Discussion
The NIVOPOSTOP study represents a significant step forward in the postoperative management of high-risk HNSCC. While adjuvant CRT improves local control, a substantial proportion of patients still develop distant metastases. The addition of nivolumab appears to provide systemic protection, extending DFS and potentially delaying or preventing recurrence.
These results align with findings from other tumor types, where adjuvant PD-1 blockade has demonstrated benefit (e.g., non-small cell lung cancer in the IMpower010 trial and melanoma in CheckMate 238). The rationale lies in the reactivation of T cells after cytoreduction and antigen release from prior treatments, including radiation-induced immunogenic cell death (Sharabi et al., 2015). If confirmed with mature OS data, this trial may establish adjuvant immunotherapy as a new standard of care in resected high-risk HNSCC.
Head and Neck Cancers: Background and Standard Treatment Before NIVOPOSTOP
Head and neck squamous cell carcinomas (HNSCC) arise from the mucosal epithelium of the oral cavity, oropharynx, hypopharynx, and larynx. These cancers collectively account for over 600,000 new cases annually worldwide. Most patients present with locally advanced disease (stage III–IVa), often requiring multimodal therapy. The cornerstone of curative treatment for resectable HNSCC has traditionally been surgical resection followed by adjuvant radiotherapy or chemoradiotherapy, depending on pathologic risk factors. Two high-risk features—positive surgical margins and extranodal extension (ENE)—have been consistently associated with a significantly increased risk of recurrence and death.
The standard of care for such high-risk patients was established in landmark trials (Cooper et al., 2004; Bernier et al., 2005), which demonstrated that postoperative radiotherapy with concurrent high-dose cisplatin chemotherapy (100 mg/m² every 3 weeks) improved locoregional control and disease-free survival compared to radiotherapy alone. This regimen became the gold standard for over two decades. However, despite aggressive CRT, recurrence rates remained high—especially due to distant metastases—and many patients experienced treatment-related toxicities that limited further systemic therapy. No standard adjuvant systemic therapy beyond CRT was available until immunotherapy began to show promise in recurrent/metastatic settings.
The NIVOPOSTOP trial was designed to fill this gap by testing whether adjuvant PD-1 inhibition could reduce recurrence and improve survival after surgery and CRT, particularly in those with minimal residual disease but high recurrence risk.
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
