What Are the Signs That Immunotherapy Is Working?
Immunotherapy has revolutionized cancer treatment by activating the body’s immune system to recognize and destroy cancer cells, offering durable responses in cancers such as melanoma, lung, and kidney cancer. Its growing use in clinical practice underscores the importance of recognizing early signs of effectiveness—such as tumor shrinkage, biomarker changes, or symptom improvement—to guide treatment decisions. According to a study by Topalian et al. (NEJM, 2019), patients showing early immune response markers had significantly better long-term survival outcomes. This article explores key indicators, clinical signs, and monitoring tools used to assess immunotherapy response and optimize care.
How Does Immunotherapy Work?
Immunotherapy is a transformative approach in cancer treatment that harnesses the body’s immune system to recognize and eliminate cancer cells. Unlike traditional therapies that directly target tumors, immunotherapy empowers immune cells to identify and attack malignancies, offering the potential for durable responses and long-term remission.The mechanism of immunotherapy involves enhancing the immune system’s ability to detect and destroy cancer cells.This is achieved through various strategies, such as immune checkpoint inhibitors, which block proteins that suppress immune responses, and cancer vaccines that stimulate the immune system to target tumor-specific antigens. By overcoming the mechanisms that allow cancer cells to evade immune detection, immunotherapy can lead to significant tumor regression and improved patient outcomes.
Clinical studies have demonstrated the efficacy of immunotherapy in various cancers. For instance, a study published in Nature reported that combining a personalized mRNA vaccine with the checkpoint inhibitor pembrolizumab reduced the risk of melanoma recurrence or death by 49% compared to pembrolizumab alone . These findings underscore the potential of immunotherapy to provide long-lasting protection against cancer. As immunotherapy continues to evolve, ongoing research aims to identify biomarkers that predict patient response and to develop combination therapies that enhance its effectiveness. Understanding the mechanisms by which immunotherapy operates is crucial for optimizing treatment strategies and improving patient outcomes in the fight against cancer.
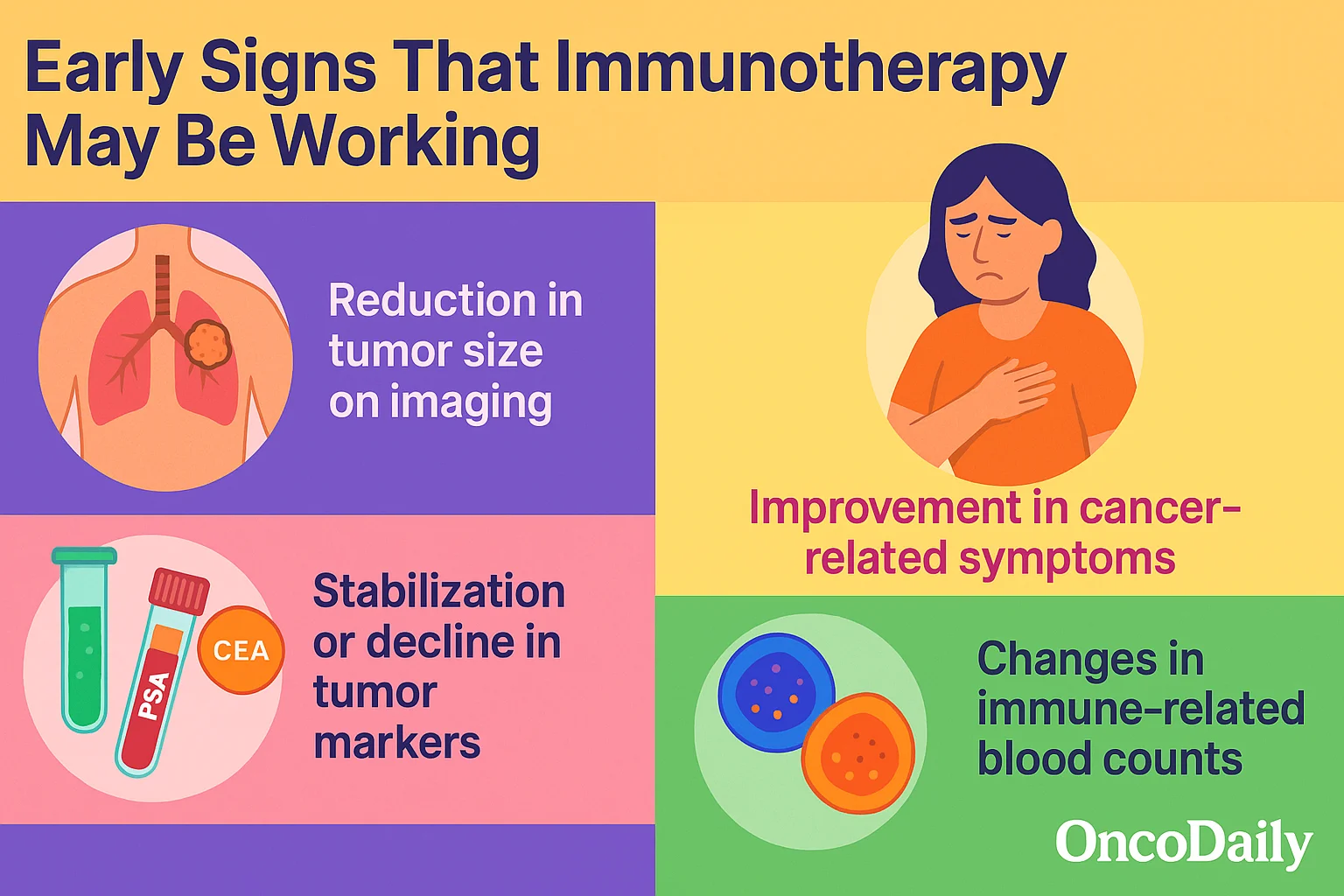
What Are the Early Signs of Immunotherapy Effectiveness?
Early signs that immunotherapy may be working include reduction in tumor size on imaging, improvement in cancer-related symptoms (such as less pain or fatigue), stabilization or decline in tumor markers (like PSA, CEA, or LDH), and changes in immune-related blood counts, such as increased lymphocyte activity or normalization of inflammatory markers. Recognizing these indicators early can help guide clinical decisions and monitor treatment response.
Changes in Tumor Size
Monitoring changes in tumor size through imaging techniques such as CT, MRI, or PET scans remains a fundamental method for assessing the effectiveness of immunotherapy. A decrease in tumor dimensions often indicates a positive response, reflecting the immune system’s successful targeting and elimination of cancer cells.
However, immunotherapy can produce unique response patterns not typically seen with conventional treatments. One such pattern is pseudoprogression, where tumors initially appear to grow or new lesions emerge before subsequent shrinkage occurs. This phenomenon is attributed to immune cell infiltration and inflammation within the tumor, temporarily increasing its size on imaging studies. Recognizing pseudoprogression is crucial to avoid prematurely discontinuing effective therapy. The iRECIST (immune Response Evaluation Criteria in Solid Tumors) guidelines have been developed to address these atypical responses, allowing for continued treatment and follow-up imaging to confirm true disease progression or response .
Additionally, advanced imaging modalities and biomarkers are being explored to differentiate between true progression and pseudoprogression. For instance, PET scans assessing metabolic activity and circulating tumor DNA (ctDNA) levels in the blood may provide complementary information to traditional imaging, aiding in more accurate response evaluation.
Symptom Relief
A reduction in cancer-related symptoms—such as pain, fatigue, and shortness of breath—can serve as an early clinical sign that immunotherapy is exerting a therapeutic effect. These improvements often correlate with underlying tumor regression or modulation of the tumor microenvironment.
- Pain Reduction: Pain is a prevalent and distressing symptom among cancer patients. Effective immunotherapy can lead to tumor shrinkage or decreased inflammatory responses, resulting in alleviated pain levels. For instance, in a study assessing symptom severity during immunotherapy treatment, patients reported a decrease in pain scores over time, suggesting a positive response to therapy.
- Fatigue Improvement: Fatigue is one of the most common adverse effects associated with cancer and its treatments. However, successful immunotherapy can lead to improved energy levels and reduced fatigue. A meta-analysis comparing fatigue incidence in patients treated with immune checkpoint inhibitors (CPIs) versus chemotherapy found that fatigue was significantly more common in chemotherapy-treated patients, indicating a better tolerability profile for immunotherapy.
- Enhanced Physical Activity: Improvements in symptoms like pain and fatigue often translate to increased physical activity and better quality of life. A study involving 629 cancer patients found that those experiencing less fatigue and pain were more likely to maintain or increase their physical activity levels, highlighting the functional benefits of symptom relief during treatment.(“The association between fatigue and pain symptoms and decreased physical activity after cancer” by S. E. Salerno et al., published in Supportive Care in Cancer in 2018.)
Improved Lab Results
Monitoring changes in blood-based biomarkers provides valuable insights into the effectiveness of immunotherapy in cancer treatment. These biomarkers, including circulating tumor DNA (ctDNA), tumor mutational burden (TMB), and specific protein levels, can reflect how well a patient’s immune system is responding to therapy.
- Circulating Tumor DNA (ctDNA): ctDNA consists of small fragments of DNA shed by tumor cells into the bloodstream. A decrease in ctDNA levels during treatment often correlates with tumor regression and improved patient outcomes. For instance, a study published in Nature Medicine demonstrated that early reductions in ctDNA levels were associated with better responses to immunotherapy in patients with non-small cell lung cancer (NSCLC) .
- Tumor Mutational Burden (TMB): TMB refers to the total number of mutations within a tumor’s genome. Higher TMB levels have been linked to increased neoantigen production, enhancing the immune system’s ability to recognize and attack cancer cells. Research has shown that patients with high TMB are more likely to respond favorably to immune checkpoint inhibitors .
- Protein Biomarkers: Certain protein levels in the blood can serve as indicators of immunotherapy response. For example, elevated levels of interleukin-8 (IL-8) have been associated with poorer outcomes, while decreasing IL-8 levels during treatment may suggest a positive response. A study in Annals of Oncology found that changes in serum IL-8 levels could predict responses to anti-PD-1 therapy in melanoma and NSCLC patients .
Incorporating these biomarker assessments into clinical practice allows for more personalized treatment strategies, enabling clinicians to monitor therapy effectiveness and make informed decisions about continuing or adjusting immunotherapy regimens.
What Are the Long-Term Signs That Immunotherapy Is Working?
Immunotherapy has revolutionized cancer treatment by offering not only immediate tumor regression but also durable long-term benefits. Key indicators of long-term success include sustained remission, improved quality of life (QoL), and extended overall survival (OS).
- Sustained Remission: Achieving and maintaining remission over extended periods is a hallmark of effective immunotherapy. For instance, in the CheckMate 067 trial, patients with advanced melanoma treated with a combination of nivolumab and ipilimumab exhibited a median overall survival of 71.9 months (approximately six years), highlighting the potential for long-term disease control .
- Improved Quality of Life: Beyond survival, immunotherapy has been associated with enhancements in patients’ QoL. A study published in CA: A Cancer Journal for Clinicians reported that patients receiving immunotherapy experienced fewer severe treatment-related adverse events compared to those undergoing chemotherapy (23% vs. 48%), suggesting a more favorable side effect profile .
- Prolonged Survival: Long-term survival benefits have been observed across various cancer types. For example, in patients with advanced non-small cell lung cancer (NSCLC), the introduction of immunotherapy has led to significant improvements in survival rates.A study comparing patients treated in the immunotherapy era (2015–2020) to those in the pre-immunotherapy era (2010–2014) found increased one-, three-, and five-year survival rates .
These findings underscore the transformative impact of immunotherapy on long-term cancer outcomes, offering hope for sustained remission, enhanced quality of life, and extended survival for many patients.
Sustained Remission
Achieving and maintaining remission over time is a critical marker of successful cancer therapy. Sustained remission indicates that the cancer remains undetectable and under control, often correlating with improved survival rates and quality of life.
Remission refers to a decrease or disappearance of signs and symptoms of cancer. According to the American Cancer Society, remission can be partial or complete. In complete remission, all signs and symptoms of cancer have disappeared, and cancer cells cannot be detected by any tests. However, it’s important to note that remission does not always equate to a cure, as cancer can return after a period of remission . Long-term remission is associated with prolonged survival in many cancer types. For instance, in acute myeloid leukemia (AML), up to half of the patients who achieve remission and undergo consolidation therapy remain in long-term remission and may be cured. This outcome is influenced by various prognostic factors, including age, overall health, and specific genetic markers .
Similarly, in early-stage breast cancer, advancements in treatment and early detection have significantly improved survival rates. A study analyzing data from the National Cancer Registration and Analysis Service in the U.K. found that the average risk of dying within five years of diagnosis decreased from 14% in the 1990s to 5% in recent years. This improvement is attributed to better treatments and increased screening efforts. Maintaining remission not only extends survival but also enhances the quality of life for cancer survivors. The American Cancer Society emphasizes the importance of managing long-term health concerns and promoting healthy behaviors to optimize wellness during survivorship.
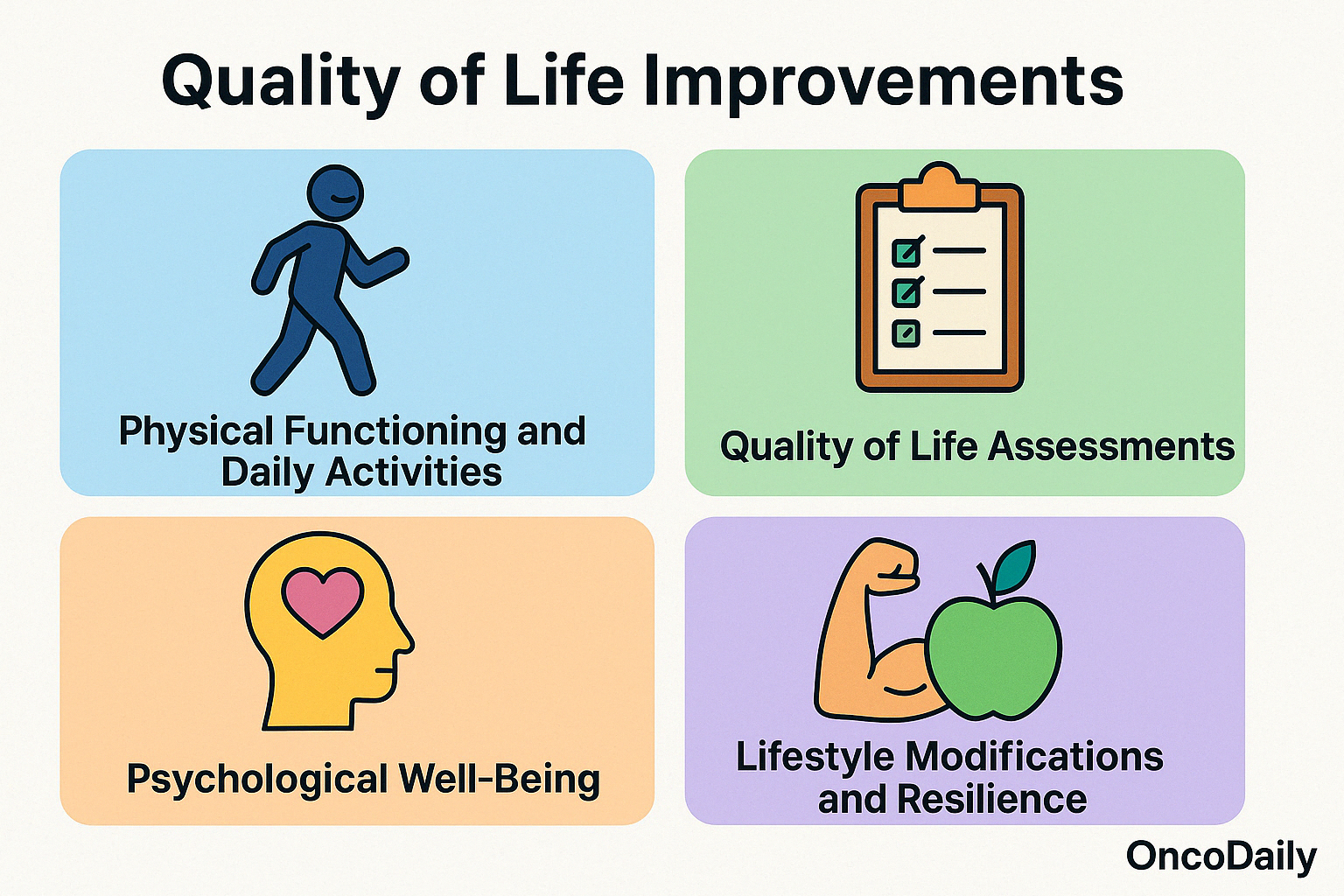
Quality of Life Improvements
Improvements in daily functioning and overall well-being are critical indicators of successful cancer treatment. These enhancements not only reflect the effectiveness of therapeutic interventions but also signify a patient’s ability to return to normalcy and maintain a higher quality of life.
- Physical Functioning and Daily Activities: Post-treatment, many cancer survivors experience a resurgence in physical capabilities, enabling them to engage in routine activities and work-related tasks. The Karnofsky Performance Status (KPS) and the Eastern Cooperative Oncology Group (ECOG) scales are commonly used to assess a patient’s functional status, with higher scores indicating better ability to carry out daily activities. Improvements in these scores post-treatment are associated with better prognosis and overall survival.
- Quality of Life Assessments: Health-Related Quality of Life (HRQoL) assessments provide comprehensive insights into a patient’s well-being. Tools like the Functional Assessment of Cancer Therapy-General (FACT-G) questionnaire evaluate physical, social, emotional, and functional well-being. Studies have shown that higher HRQoL scores correlate with improved treatment outcomes and can predict survival rates in cancer patients.
- Psychological Well-Being: Psychological health is a critical component of overall well-being. Interventions focusing on mental health, such as cognitive-behavioral therapy and mindfulness-based stress reduction, have demonstrated efficacy in reducing anxiety, depression, and fatigue among cancer patients. These improvements contribute to better adherence to treatment and enhanced quality of life.
- Lifestyle Modifications and Resilience: Cancer survivors often adopt healthier lifestyles post-treatment, including increased physical activity, improved diet, and stress management techniques. Such changes not only aid in recovery but also reduce the risk of recurrence.
Prolonged Survival
One of the most compelling measures of successful cancer treatment is improved overall survival, and in recent years, immunotherapy has emerged as a driving force behind this progress across multiple malignancies. By restoring or enhancing the body’s ability to recognize and destroy cancer cells, immunotherapy has led to durable remissions and significantly extended life expectancy in cancers that were once considered largely incurable.
In non–small cell lung cancer (NSCLC), a large population-based study published in Cancer by Xuesong Han and colleagues (American Cancer Society, 2023) analyzed over 191,000 patients and found that the five-year overall survival increased from 6.8% before the immunotherapy era (2010–2014) to 10.7% during 2015–2020, following the introduction of immune checkpoint inhibitors like pembrolizumab and nivolumab. Median survival also improved from 7 to 8 months, despite being modest, indicating a meaningful shift in long-term outcomes attributable to immune-based therapies. Similarly, in advanced melanoma, where survival outcomes were historically poor, long-term follow-up from the CheckMate 067 trial (Larkin et al., NEJM) showed that patients treated with the combination of nivolumab and ipilimumab achieved a 10-year melanoma-specific survival rate of 52%. This is a dramatic improvement over pre-immunotherapy survival rates, where less than 10% of patients lived beyond five years. These data reinforce the potential of immunotherapy not just for temporary control, but for functional cures in a substantial proportion of patients.
Further evidence comes from a recent clinical trial led by University College London, evaluating pembrolizumab in patients with MSI-H or mismatch repair-deficient colorectal cancer. According to reports in The Times, 59% of patients had no detectable cancer after just nine weeks of treatment. This suggests not only a dramatic response rate but also the potential to reduce or even eliminate the need for surgery or chemotherapy in select patients—a shift that could transform both survival and quality of life. The American Cancer Society has documented steady improvements in cancer survival over time. As detailed in the 2025 Cancer Facts & Figures report, the five-year relative survival rate for all cancers combined has risen from 39% in the 1960s to 70% in White patients and 65% in Black patients. These advances are credited to early detection, improved therapies, and most notably, the rise of precision immuno-oncology.
What Are the Signs That Immunotherapy Is Not Working?
While immunotherapy offers promising outcomes for many patients, it is essential to recognize early indicators that treatment may not be effective. These signs can include continued tumor growth or new lesions on imaging, worsening cancer-related symptoms such as increased pain or fatigue, rising tumor markers in blood tests, lack of clinical improvement, and the development of severe immune-related adverse effects without corresponding tumor response. Recognizing these early can help guide timely reassessment and alternative treatment planning.
Tumor Growth or Spread
Increases in tumor size or the emergence of new metastases during immunotherapy can signify that the treatment is not effectively controlling the cancer. While some patients may experience initial tumor enlargement due to immune cell infiltration—a phenomenon known as pseudoprogression—true disease progression is characterized by sustained tumor growth and the appearance of new lesions over time.
- Imaging Findings: Imaging studies, such as CT or MRI scans, are essential tools for monitoring tumor response to immunotherapy. A study published in Cancer Imaging highlighted that continuous progression of liver metastases observed through imaging, without significant changes in tumor growth rate compared to baseline, may indicate treatment failure rather than pseudoprogression. In such cases, the lack of a significant change in tumor growth rate suggests that the immunotherapy is not effectively controlling the disease.
- Hyperprogressive Disease: In rare instances, immunotherapy may lead to hyperprogressive disease (HPD), where tumors grow more rapidly after treatment initiation. HPD is characterized by a sudden acceleration in tumor growth, often leading to a poor clinical outcome. A review in Oncodaily discussed that HPD can occur shortly after starting immune checkpoint inhibitors and underscores the importance of careful patient selection and monitoring during immunotherapy.
- Clinical Implications: When imaging reveals sustained tumor growth or new metastases, clinicians may consider alternative treatment strategies.Options include switching to different immunotherapeutic agents, combining immunotherapy with other modalities like chemotherapy or targeted therapy, or enrolling the patient in clinical trials exploring novel treatments. The decision should be individualized based on the patient’s overall health, cancer type, and prior treatment history.
Worsening Symptoms
While immunotherapy has revolutionized cancer treatment, offering hope for many patients, it’s crucial to recognize when it’s not yielding the desired outcomes. An increase in cancer-related symptoms, such as pain and fatigue, can be indicative of treatment failure.
Fatigue is a common side effect of immunotherapy. However, when fatigue becomes progressively worse or doesn’t improve over time, it may signal that the treatment isn’t effectively controlling the disease. A study published in Cancer Imaging highlighted that fatigue was among the most frequently reported symptoms during immunotherapy, with its persistence potentially indicating a lack of therapeutic response. An escalation in pain, especially if localized to known tumor sites, can suggest tumor progression despite ongoing immunotherapy. The American Cancer Society notes that worsening pain may be a sign that the cancer is not responding to treatment, necessitating a reassessment of the therapeutic approach.
Clinicians monitor patients closely for changes in symptomatology. If a patient reports increased fatigue or pain, it prompts further diagnostic evaluations, such as imaging studies or biomarker assessments, to determine disease progression. Based on these findings, oncologists may consider modifying the treatment regimen, exploring alternative therapies, or enrolling the patient in clinical trials to identify more effective interventions.
Adverse Reactions
While immunotherapy has revolutionized the landscape of cancer treatment, offering durable responses and extended survival in many cancers, it is not without risk. Unlike traditional chemotherapy, which acts directly on rapidly dividing cells, immunotherapy works by unleashing the immune system—sometimes with unintended consequences. In certain cases, a patient’s own immune system can begin to attack healthy tissues, resulting in immune-related adverse events (irAEs). When severe, these reactions may not only impact quality of life but also suggest that the treatment may not be well-tolerated or effective in that patient.
One of the most telling signs of a potential issue is the development of immune-mediated inflammation in critical organs. For example, checkpoint inhibitors targeting PD-1, PD-L1, or CTLA-4 pathways have been associated with serious conditions such as colitis, pneumonitis, hepatitis, and endocrinopathies. These adverse events can manifest with symptoms such as severe diarrhea, persistent cough, jaundice, or unexplained fatigue. If not recognized and managed quickly, they can escalate into life-threatening complications. In clinical observations, colitis is one of the more frequent severe irAEs, often presenting with abdominal pain and bloody diarrhea. If left untreated, it can lead to bowel perforation. Similarly, immune-related pneumonitis can result in respiratory failure, and autoimmune hepatitis may progress to liver failure if not promptly addressed. A study published in the Journal of Clinical Oncology by Naing et al. (2021) emphasized that the early use of corticosteroids is essential to mitigate these reactions and, in many cases, allows for the safe continuation of treatment (Journal of Clinical Oncology, American Society of Clinical Oncology).
The standard management of severe irAEs includes high-dose systemic corticosteroids, such as prednisone or methylprednisolone. If the symptoms are refractory to steroids, second-line immunosuppressants like infliximab or mycophenolate mofetil may be required. Importantly, if symptoms persist or worsen despite these interventions, immunotherapy may need to be permanently discontinued. The American Cancer Society also emphasizes that severe immune reactions—especially when persistent—might indicate that the risks of continuing treatment outweigh the benefits. In such scenarios, oncologists often re-evaluate the treatment plan and consider alternative therapies that are better tolerated.
How Is the Effectiveness of Immunotherapy Monitored?
Monitoring the effectiveness of immunotherapy requires a combination of clinical tools that offer both structural and biological insight. The most commonly used methods include imaging tests—such as CT, MRI, and PET scans—to assess changes in tumor size and spread; blood tests, which track biomarkers like tumor markers or circulating tumor DNA to reflect treatment response at the molecular level; and physical examinations, where physicians evaluate symptoms, performance status, and visible changes. Together, these approaches help clinicians determine whether immunotherapy is working, detect complications early, and make informed decisions about continuing or adjusting treatment.
Imaging Tests
Imaging tests are essential tools for evaluating how well a patient is responding to immunotherapy. They offer visual, structural, and functional data that help clinicians assess whether tumors are shrinking, stable, or progressing—key indicators of treatment success or failure.
Computed tomography (CT) scans are the most commonly used imaging modality in cancer care. They provide detailed cross-sectional views of the body and are typically performed every 8 to 12 weeks during treatment to track changes in tumor size. In immunotherapy, CT scans help monitor not only the primary tumor but also detect new lesions that may indicate disease progression. However, interpreting CT scans in this context can be complex due to phenomena like pseudoprogression, where tumors appear larger before shrinking as immune cells infiltrate the tumor. Magnetic resonance imaging (MRI) is particularly useful for assessing soft tissue tumors and metastases in the brain, liver, and spine. In immunotherapy, MRIs are often used when tumors are located in anatomically sensitive areas where structural detail is crucial. For example, patients with melanoma brain metastases may undergo regular brain MRIs every 2 to 3 months to monitor treatment response and detect inflammation or edema.
Positron emission tomography (PET) scans, often combined with CT (PET/CT), provide functional information by measuring metabolic activity in tissues. PET scans are valuable in immunotherapy because they can help distinguish between active tumor tissue and scar tissue or inflammation. A decrease in metabolic activity—even without significant size reduction—can suggest a favorable immune response. PET scans are typically used selectively, especially in cancers like Hodgkin lymphoma or in cases where anatomical imaging is inconclusive. Together, these imaging modalities guide clinicians in evaluating treatment efficacy, determining whether to continue, adjust, or stop immunotherapy, and in differentiating between true disease progression and immune-related effects. Accurate imaging interpretation—often guided by immune-specific criteria like iRECIST—is critical to ensuring the best outcomes for patients receiving immunotherapy.
Blood Tests and Biomarkers
Blood tests play a vital role in tracking the effectiveness of immunotherapy by offering insights into both tumor activityand the immune system’s response. These tests are minimally invasive and can be repeated regularly—often every few weeks—to monitor changes over time.
One of the most informative tools is the measurement of tumor markers, such as CEA in colorectal cancer, PSA in prostate cancer, or LDH in melanoma. A decline in these markers during treatment often signals a positive response, while rising levels may indicate tumor progression or resistance. Another valuable indicator is circulating tumor DNA (ctDNA)—tiny fragments of DNA released by cancer cells into the bloodstream. Studies have shown that a reduction in ctDNA levels early in treatment correlates with improved survival outcomes, often before changes are visible on imaging.
In addition, routine immune panel tests can measure lymphocyte counts, inflammatory markers (e.g., CRP, IL-6), or cytokine profiles, which reflect immune activation or suppression. Persistent lymphopenia or abnormal cytokine levels may point to immune exhaustion or an inadequate response. These blood-based biomarkers help clinicians tailor treatment decisions, assess toxicity risk, and detect disease recurrence earlier—making them a critical complement to imaging and clinical assessment in immunotherapy monitoring.
Physical Exams and Patient Feedback
Regular physical exams and patient-reported outcomes are essential tools in evaluating immunotherapy progress. During clinic visits, doctors assess vital signs, weight changes, skin condition, lymph node size, and any new or worsening symptoms such as shortness of breath, pain, or neurological changes. These physical findings help detect complications like pneumonitis, colitis, or endocrine dysfunctions that may not yet appear on scans.
Equally important are the patient’s own reports. Clinicians often use standardized questionnaires or simply engage in structured dialogue to understand changes in energy levels, appetite, pain, and daily functioning. A decline in these areas can signal disease progression or treatment-related side effects, while stability or improvement may support continuing the current regimen.
What Should Patients Discuss with Their Doctors?
Open communication with your healthcare provider is essential during immunotherapy. Regular conversations help ensure that your treatment is effective, side effects are managed early, and your expectations are realistic and informed. Below is a checklist of important questions and discussion points to bring up during appointments:
-
How is my cancer responding to treatment?
-
Are there signs of side effects or immune-related complications?
-
Should I be concerned about changes in pain, appetite, or energy levels?
-
What should I monitor at home between appointments?
-
How often will tests or scans be done to track progress?
-
What happens if the treatment stops working?
-
What lifestyle changes can support my treatment?
-
Are there long-term effects I should prepare for?
You Can Watch More on OncoDaily Youtube TV
Written by Toma Oganezova, MD
FAQ
What are the first signs that immunotherapy is working?
Early signs include tumor shrinkage on scans, symptom relief (like less pain or fatigue), improved lab results (such as lower tumor markers), and increased energy levels.
How long does it take to know if immunotherapy is effective?
Response times vary by cancer type and individual, but initial effects are usually assessed within 6–12 weeks through imaging and blood tests.
Can symptoms improve even if the tumor doesn’t shrink?
Yes. Some patients feel better due to reduced inflammation or immune response, even if scans don’t show immediate tumor shrinkage.
What is pseudoprogression in immunotherapy?
Pseudoprogression refers to temporary tumor swelling due to immune cell infiltration. Tumors may appear to grow before shrinking—this doesn’t mean treatment has failed.
What are signs that immunotherapy is not working?
Persistent or worsening symptoms, tumor growth on scans, rising tumor markers, and lack of symptom improvement may indicate the therapy is ineffective.
Are there long-term signs that immunotherapy is successful?
Yes. These include sustained remission, improved physical functioning and energy, fewer or stable cancer-related symptoms, and longer overall survival.
How is immunotherapy effectiveness monitored?
Through CT/MRI/PET scans, blood tests (e.g., ctDNA, tumor markers), physical exams, and patient-reported symptoms.
What should I ask my doctor about treatment progress?
Discuss scan results, symptom changes, lab tests, side effects, expected outcomes, and what to watch for at home.
Can immunotherapy work even if chemotherapy didn’t?
Yes. Immunotherapy works differently and can benefit patients whose cancers did not respond to chemo, especially if the tumor has MSI-H or high TMB.
Does developing side effects mean immunotherapy is working?
Not always, but some immune-related side effects (like thyroid inflammation) have been associated with better outcomes in certain studies.
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
