New data from the Phase 1 CUE-101-01 trial (NCT03978689) highlight encouraging clinical activity for CUE-101, a novel HPV16-directed immunotherapy, when combined with pembrolizumab (Keytruda) in the first-line treatment of recurrent or metastatic HPV-positive head and neck squamous cell carcinoma (HNSCC). A confirmed overall response rate of 50% was observed with the combination therapy, with early evidence of prolonged survival and disease control, according to reporting by Jordyn Sava and review by Sabrina Serani.
The updated results, based on data as of July 14, 2025, come from the expansion cohort of the Phase 1 trial. Two patients had confirmed complete responses (CRs), with an additional CR reported in a patient who initially showed prolonged stable disease—bringing the total to 2 CRs. Partial responses (PRs) were observed in 10 additional patients.
Notably, responses were observed even in patients with low PD-L1 expression (combined positive score [CPS] 1–19), a subgroup typically less responsive to immune checkpoint inhibition. The 12-month overall survival rate was 88%, with median overall survival reaching 32 months in this early cohort, suggesting a potentially meaningful benefit.
What Does HPV-Positive Mean and Why Does It Matter for Immunotherapy?
HPV-positive refers to cancers that are driven by the human papillomavirus (HPV)—most commonly HPV16 in head and neck squamous cell carcinoma (HNSCC). These tumors often express viral antigens that the immune system can recognize, making them more immunogenic than HPV-negative tumors.
Because of this, HPV-positive cancers tend to respond better to immunotherapy, especially when treatments like checkpoint inhibitors or antigen-specific therapies are used to enhance T cell recognition and killing of virally driven tumor cells.
A Closer Look at CUE-101
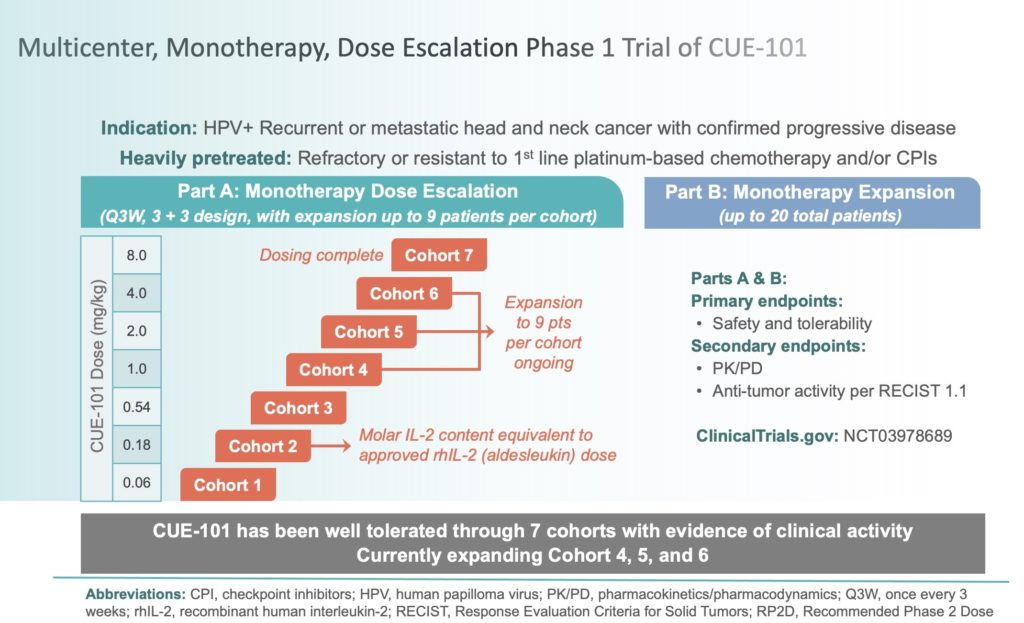
CUE-101 is a modular T-cell engager developed using Cue Biopharma’s Immuno-STAT™ platform. It is designed to selectively activate HPV16-specific CD8+ T cells by delivering an HLA-A*0201–restricted HPV16 E7 peptide fused to attenuated IL-2. The goal is to drive tumor-specific T-cell activation while avoiding systemic cytokine toxicity often associated with non-specific IL-2 therapies.
In combination with pembrolizumab, CUE-101 is hypothesized to amplify antigen-specific immune responses and improve checkpoint blockade effectiveness in HPV-associated cancers.
“We are excited to report an additional CR in a patient with recurrent metastatic HPV-positive HNSCC treated with CUE-101 in combination with pembrolizumab,” said Matteo Levisetti, MD, Chief Medical Officer at Cue Biopharma.
“This patient had multiple disease sites, including the lungs, that cleared prior to the complete response seen in the primary lesion. The durable kinetics reflect how repeated tumor-specific T-cell engagement may offer a distinct clinical trajectory compared to traditional cytotoxic therapies.”
How It Compares
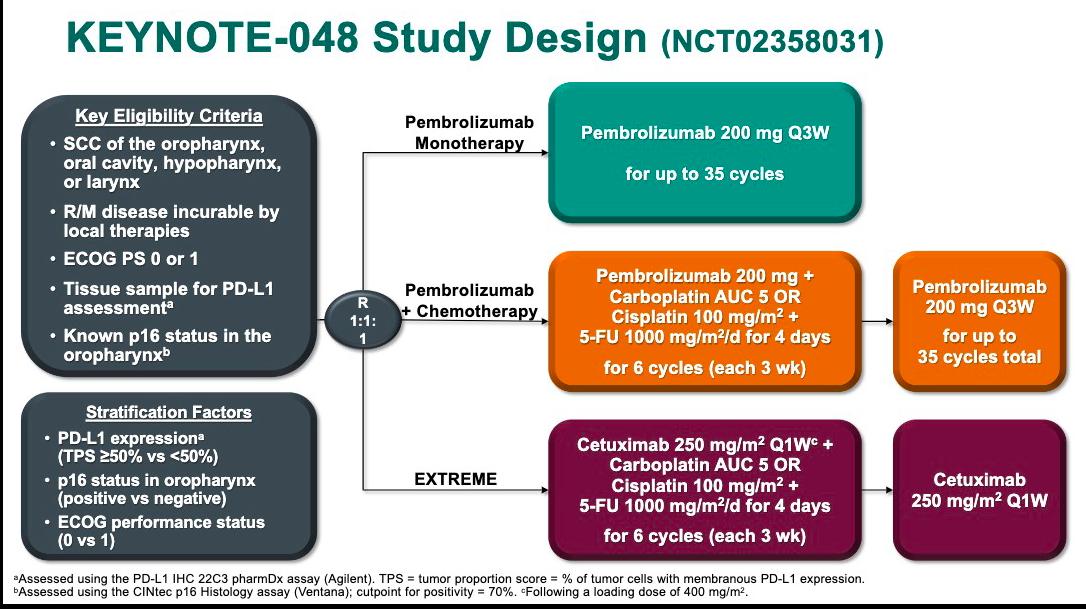
The results compare favorably to KEYNOTE-048 (NCT02358031), where pembrolizumab monotherapy in a similar first-line setting resulted in a 19% response rate, a 12.3-month median OS, and 57% 12-month OS. While cross-trial comparisons have limitations, the 50% response rate and 32-month median OS seen with the CUE-101 combination underscore its potential impact.
In addition to first-line efficacy, CUE-101 monotherapy in the second-line setting also showed promising activity. Among patients previously treated with chemotherapy or immunotherapy, median OS was 20.8 months, with a 12-month OS rate of 59%. At a separate 2023 data cutoff, 20 of 22 patients treated with second-line CUE-101 + pembrolizumab were alive, and 13 remained on treatment.
Safety and Development Pathway
The safety profile of the combination was manageable. Most adverse events were grade 1 or 2, consistent with known profiles of pembrolizumab and IL-2–based agents. Grade ≥3 treatment-related events were infrequent and manageable, supporting continued development.
The trial enrolled 27 patients in the first-line combination cohort, all of whom had HPV16-positive, HLA-A*0201–restricted tumors with PD-L1 CPS ≥1. CUE-101 was administered every three weeks alongside standard pembrolizumab dosing (200 mg Q3W). A randomized Phase 2 trial is planned to confirm the magnitude of clinical benefit and support potential regulatory discussions.
Summary
The combination of CUE-101 + pembrolizumab demonstrates strong preliminary efficacy in a population with historically limited response rates to immunotherapy. With 50% response rate, 32-month median OS, and favorable safety, these data position the CUE-101 platform as a potentially transformative immunotherapy for HPV-driven cancers. Further study is ongoing.
You Can Watch More on OncoDaily Youtube TV


