The CheckMate 8HW study (NCT04008030) evaluated the combination of nivolumab (NIVO) and ipilimumab (IPI) in patients with microsatellite instability-high (MSI-H) or mismatch repair-deficient (dMMR) metastatic colorectal cancer (mCRC). Presented by Dr. Elena Elez Fernandez from Barcelona, Spain, at ESMO GI 2025, the trial demonstrated significant improvements in progression-free survival (PFS) for NIVO + IPI compared to both chemotherapy and NIVO alone. This report focuses on the health-related quality of life (HRQoL) outcomes, comparing NIVO + IPI with NIVO alone, to assess the impact of this combination on patient-reported symptoms, functioning, and overall well-being.

Background
The CheckMate 8HW study (NCT04008030) demonstrated that the combination of nivolumab (NIVO) and ipilimumab (IPI) significantly improved progression-free survival (PFS) compared to chemotherapy and NIVO alone in patients with microsatellite instability-high (MSI-H) or mismatch repair-deficient (dMMR) metastatic colorectal cancer (mCRC). The dual primary endpoints of PFS for NIVO + IPI versus chemotherapy (hazard ratio [HR], 0.21; [97.91% CI, 0.13–0.35]; P < 0.0001) and NIVO + IPI versus NIVO across all lines (HR, 0.62 [95% CI, 0.48–0.81]; P = 0.0003) were met. In this report, we focus on the health-related quality of life (HRQoL) analyses comparing NIVO + IPI with NIVO alone in these patients.
Methods
HRQoL analyses were conducted in patients with centrally confirmed MSI-H/dMMR mCRC who had received at least one dose of NIVO + IPI or NIVO alone. Descriptive analyses assessed mean scores and changes from baseline (BL) using the EORTC QLQ-C30/QLQ-C29 and the EQ-5D-3L questionnaires. These tools evaluate overall health status, functioning, symptoms, and health utility. The data were collected at baseline and throughout the treatment period, with analysis of changes in various clinically relevant scales including global health status (GHS), physical functioning, fatigue, diarrhea, and pain.
Results
The results from the CheckMate 8HW study (NCT04008030) presented by Dr. Elena Elez Fernandez at ESMO GI 2025 highlight the impact of nivolumab (NIVO) and ipilimumab (IPI) combination therapy on health-related quality of life (HRQoL) in patients with MSI-H/dMMR mCRC. The following findings provide insights into the improvements in symptoms, functioning, and overall patient well-being with NIVO + IPI compared to NIVO alone.
Patient Demographics and Completion Rates:
- Of 582 patients treated with either NIVO + IPI (n = 296) or NIVO (n = 286), questionnaire completion rates were greater than 90% at baseline and over 80% at all on-treatment visits with at least 10 patients completing each instrument.
- Median follow-up was 47.0 months (range, 16.7–60.5).
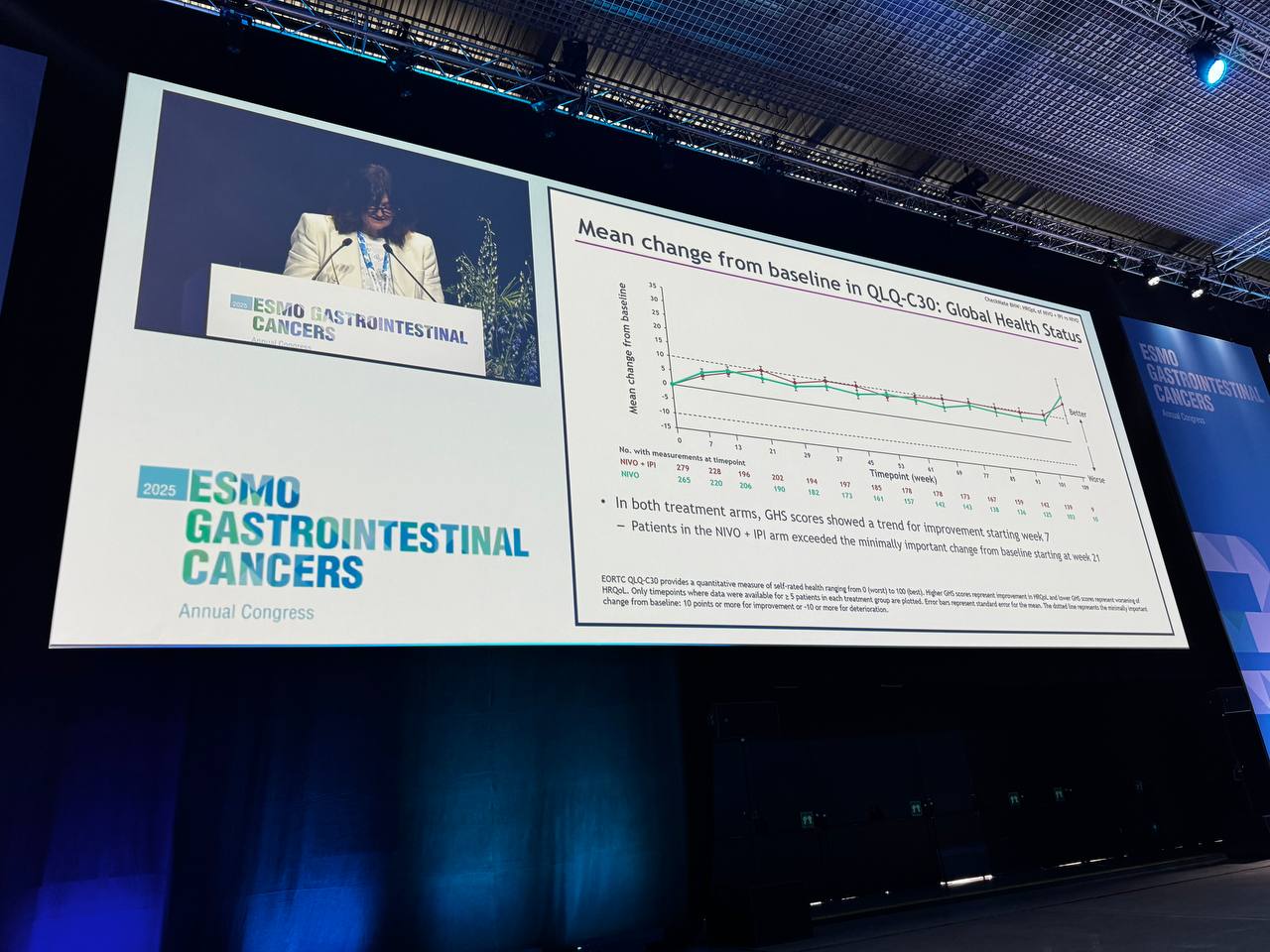
Global Health Status (GHS):
- NIVO + IPI: Mean change from baseline (BL): 10.1 (95% CI, 7.0–13.1)
- NIVO alone: Mean change from BL: 7.1 (95% CI, 4.2–10.1)
- Significant improvement with NIVO + IPI, surpassing the minimally important change (MIC) from BL starting at week 21.
Physical Functioning:
- NIVO + IPI: 6.5 (95% CI, 4.0–8.9)
- NIVO alone: 3.5 (95% CI, 1.1–5.9)
Fatigue:
- NIVO + IPI: -10.4 (95% CI, -13.9 to -7.0)
- NIVO alone: -7.3 (95% CI, -10.6 to -4.1)
- Significant improvement in fatigue with NIVO + IPI starting at week 21, surpassing MIC.
Diarrhea:
- NIVO + IPI: -3.8 (95% CI, -7.3 to -0.3)
- NIVO alone: -2.1 (95% CI, -6.2 to 2.0)
Pain:
- NIVO + IPI: -15.4 (95% CI, -19.6 to -11.3)
- NIVO alone: -10.1 (95% CI, -13.9 to -6.3)
- Significant improvement with NIVO + IPI in pain reduction from BL.
Visual Analogue Scale (VAS):
- NIVO + IPI: 9.8 (95% CI, 6.8–12.8)
- NIVO alone: 5.8 (95% CI, 2.8–8.7)
- Significant improvement in VAS with NIVO + IPI starting at week 21.
Utility Index (UI):
- NIVO + IPI: 0.077 (95% CI, 0.043–0.111)
- NIVO alone: 0.074 (95% CI, 0.038–0.109)
Conclusions
NIVO + IPI demonstrated clinically meaningful and statistically significant improvements in health-related quality of life (HRQoL) compared to NIVO alone in patients with centrally confirmed MSI-H/dMMR mCRC. Improvements in key symptom scales such as pain, fatigue, and global health status were observed, with NIVO + IPI providing better symptom control and QoL without compromising survival benefits. The addition of IPI to NIVO significantly improved progression-free survival (PFS) while maintaining a favorable HRQoL profile, supporting NIVO + IPI as a new therapeutic option in MSI-H/dMMR mCRC.
You can read the full abstract here


