ESMO GI 2025 Highlights: SIERRA Study on STRIDE in Unresectable HCC
SIERRA study (NCT05883644) was introduced by Dr. Stephen L. Chan from Sha Tin, Hong Kong SAR China, during the ESMO GI 2025 conference. The trial investigates the safety and efficacy of STRIDE (Single T Regular Interval D) as a first-line treatment for unresectable hepatocellular carcinoma (HCC) in a broader patient population with poorer prognoses compared to the HIMALAYA study (NCT03298451).
This includes patients with Child-Pugh B7/B8, ECOG performance status 2, or chronic main trunk portal vein thrombosis. Dr. Chan’s presentation provided important insights into the safety profile of STRIDE, highlighting its potential as a treatment option for patients with advanced HCC and poor prognoses.
Background
The phase 3b SIERRA study (NCT05883644) is designed to evaluate the safety and efficacy of STRIDE (Single T Regular Interval D) as a first-line treatment (1L tx) for patients with unresectable hepatocellular carcinoma (HCC). This trial is particularly significant as it includes a broader and more challenging patient population compared to the HIMALAYA study (NCT03298451).
The SIERRA study specifically targets patients with poorer prognoses, such as those with Child-Pugh (CP) B7/B8, ECOG performance status (PS) 2, or chronic main trunk portal vein thrombosis (Vp4). These patients are often excluded from clinical trials, making this study especially valuable for assessing how STRIDE performs in a high-risk cohort with advanced liver disease and other complicating factors.
Methods
In the SIERRA study, patients receive an initial combination therapy consisting of 1500 mg of durvalumab (D) and 300 mg of tremelimumab (T) on Day 1. Following this initial dose, patients are treated with durvalumab monotherapy at 1500 mg every 4 weeks. This treatment regimen combines immune checkpoint inhibitors, durvalumab and tremelimumab, to potentially enhance the immune response against HCC.
The co-primary endpoints of the study focus on safety and efficacy. The safety endpoint assesses the incidence of Grade 3 or 4 potentially treatment-related adverse events (PRAEs) within the first 6 months of treatment. This measure focuses on the occurrence of serious adverse events that may be associated with the STRIDE regimen, especially those that could lead to treatment discontinuation or significant clinical issues. The second co-primary endpoint is the objective response rate (ORR), which evaluates the proportion of patients who experience significant tumor shrinkage or complete responses following treatment.
To ensure a thorough safety and efficacy evaluation, the safety data were reviewed once approximately 60 patients had been followed for at least 6 months. This time frame allows for detailed monitoring of treatment effects, providing a clear picture of how the therapy performs over time. The data cut-off date for this safety analysis was set as September 27, 2024, which marks the point at which the results were compiled for review.
Results
By the data cut-off, 98 patients had received at least one dose of the study treatment. This group included 35 patients with CP B7/B8, 44 patients with ECOG PS 2, and 19 patients with Vp4. The median age was 70 years, and 87% of the patients were male. The median number of treatment cycles ranged from 2.0 for CP B7/B8 patients to 7.0 for those with ECOG PS 2.
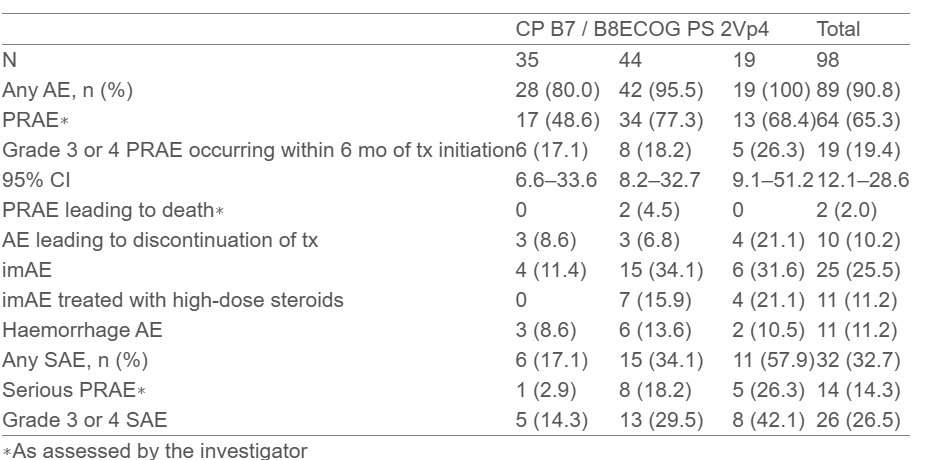
- Grade 3 or 4 PRAEs occurred in 19.4% of patients (95% CI, 12.1–28.6).
- Any AE was reported in 90.8% of patients, with 65.3% experiencing PRAEs.
- Serious AEs occurred in 32.7% of patients, with serious PRAEs in 14.3%.
- Immune-mediated AEs (imAEs) were reported in 25.5% of patients, with some requiring high-dose steroids.
Safety by Subgroup:
- CP B7/B8: 80% experienced any AE, 48.6% had PRAEs, and 17.1% had Grade 3/4 PRAEs.
- ECOG PS 2: 95.5% experienced any AE, 77.3% had PRAEs, and 18.2% had Grade 3/4 PRAEs.
- Vp4: 100% experienced any AE, 68.4% had PRAEs, and 26.3% had Grade 3/4 PRAEs.
Conclusions
The safety profile of STRIDE was manageable and aligned with that observed in the HIMALAYA study, despite enrolling patients with poorer hepatic function, worse ECOG performance status, and more advanced vascular invasion. These findings support the potential of STRIDE as a treatment option for patients with unresectable HCC and poor prognoses.
You can read the full article here.
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
