Zanzalintinib, an investigational next-generation tyrosine kinase inhibitor, significantly improved overall survival when combined with the PD-L1 inhibitor atezolizumab compared to regorafenib in the Phase 3 STELLAR-303 trial. The study enrolled patients with previously treated metastatic colorectal cancer (mCRC)—a population with limited effective treatment options.
Alameda, Calif. – June 22, 2025 – Exelixis, Inc. (Nasdaq: EXEL) announced that the Phase 3 STELLAR-303 trial met its primary endpoint, demonstrating a statistically significant improvement in overall survival (OS) with the combination of zanzalintinib (XL092) and atezolizumab compared to regorafenib in patients with previously treated metastatic colorectal cancer (mCRC).

About Exelixis
Exelixis is an oncology-focused biotechnology company committed to developing therapies that improve outcomes for patients with cancer. The company’s pipeline includes a range of novel small molecule therapies and strategic partnerships with leading immuno-oncology platforms.
For more information, visit www.exelixis.com
Colorectal Cancer Worldwide
Colorectal cancer (CRC) arises in the colon or rectum and is among the most common and deadly cancers worldwide, with over 1.9 million new cases and nearly 930,000 deaths in 2020.
It progresses slowly—typically over a decade—from benign polyps to malignant tumors, influenced by factors such as age, genetics, diet, obesity, and smoking . Early-stage disease is often symptomless, underscoring the importance of screening, while tumor location (right vs. left colon) affects its biology and prognosis, with right-sided tumors generally having more aggressive features and poorer outcomes
What is zanzalintinib and how does it work?
Zanzalintinib (XL092) is a cutting-edge, multi-targeted tyrosine kinase inhibitor primarily directed against VEGFR, with additional immunomodulatory actions. This comprehensive review summarizes both preclinical and early clinical findings, and outlines future prospects for zanzalintinib—especially when combined with immune checkpoint inhibitors (ICIs).
Preclinical insights suggest that zanzalintinib’s inhibition of MET and TAM kinases may enhance immune responses, supporting its synergy with ICIs. Early clinical trials, including a phase 1 dose-escalation study, have demonstrated that pairing zanzalintinib with an anti–PD-L1 agent is well tolerated, without unexpected toxicities.
Currently, several ongoing clinical studies are evaluating this combination across multiple solid tumors—such as renal cell carcinoma, colorectal cancer, and head and neck cancer—with some advancing to phase 3. While this strategy holds significant therapeutic promise, identifying reliable biomarkers to predict which patients will benefit from the anti-angiogenesis and ICI combo remains a key challenge due to the complexity of their combined mechanisms
STELLAR-303 Trial
STELLAR‑303 is a global, open-label, randomized phase III trial comparing zanzalintinib (XL092) 100 mg daily plus atezolizumab (1,200 mg every 3 weeks) versus regorafenib (160 mg on days 1–21 of each 28-day cycle) in patients with microsatellite-stable or MSI-low metastatic colorectal cancer (mCRC) that progressed after standard therapies
The study plans to enroll approximately 874–901 adult patients with measurable mCRC and known RAS status who have received all SOC regimens (fluoropyrimidine, irinotecan, oxaliplatin ± anti-VEGF, anti-EGFR if RAS wild-type, and BRAF-targeted therapy if applicable). Enrollment for patients with non-liver metastases (NLM) is capped at ~350, and ~524 patients with liver metastases will also be included
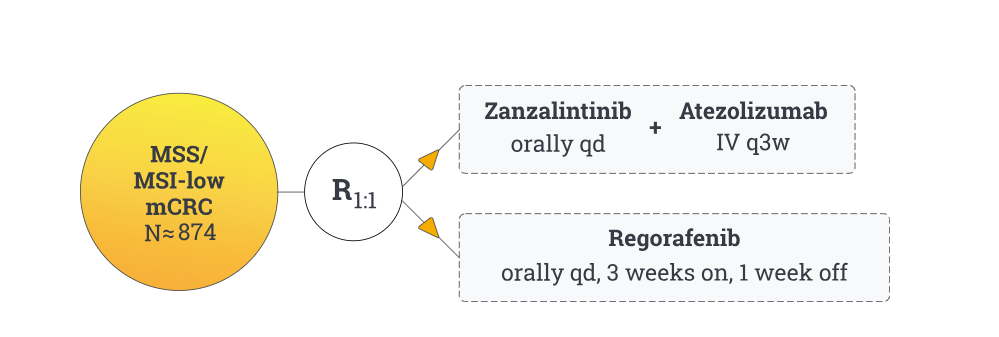
The study has two co-primary endpoints:
- Overall survival (OS) in the intent-to-treat population
- OS in the non-liver metastases subgroup.
Secondary endpoints include progression-free survival (PFS), objective response rate (ORR), and duration of response (DOR) per investigator assessment, along with exploratory health-related quality of life measures
The STELLAR-303 study met its primary endpoint, showing a statistically significant survival benefit in the intent-to-treat population. Secondary endpoints included progression-free survival, objective response rate, duration of response, and quality-of-life measures. Safety findings were consistent with prior studies, with no new signals observed.
“The STELLAR-303 results, which showed a survival benefit with the combination of zanzalintinib and atezolizumab versus regorafenib across all randomized patients with previously treated metastatic colorectal cancer, marks an important first milestone for our zanzalintinib pivotal development program,” said Amy Peterson, M.D., Executive Vice President of Product Development & Medical Affairs and Chief Medical Officer at Exelixis. “We look forward to discussing the findings with regulatory authorities and presenting the detailed results at an upcoming medical conference.”
You can read the full press release here.
Written by Sona Karamyan, MD


