Intratumor heterogeneity is a defining feature of pancreatic ductal adenocarcinoma (PDAC), contributing to treatment resistance and poor prognosis. Published in the Journal of Pathology (2025), this study takes a bottom-up approach to redefine PDAC subtypes based on molecular profiling. By analyzing tumor samples from 95 patients, researchers identified distinct biomarker-driven subtypes, revealing significant variability within individual tumors. These findings emphasize the need for personalized therapeutic strategies and highlight the potential of molecular classification in improving patient outcomes.
Authors: Marc Hilmi, Flore Delecourt, Jérôme Raffenne, Taib Bourega, Nelson Dusetti, Juan Iovanna, Yuna Blum, Magali Richard, Cindy Neuzillet, Anne Couvelard, Matthieu Tihy, Louis de Mestier, Vinciane Rebours, Rémy Nicolle, Jérôme Cros
Published in Journal of Pathology in Feb 2025
Background
Pancreatic ductal adenocarcinoma (PDAC) remains one of the most aggressive cancers, characterized by a high degree of intratumor heterogeneity. This heterogeneity poses challenges in treatment efficacy and patient prognosis. While previous studies have aimed to identify specific biomarkers and molecular subtypes of PDAC, understanding the degree of variability within individual tumors is still limited. This study aimed to provide a deeper understanding of the molecular diversity within PDAC by analyzing tumor tissue from a large cohort of patients to identify key biomarkers and tumor subtypes, with the ultimate goal of improving prognosis and therapeutic strategies.
Methods
A cohort of 95 patients with resected PDAC were included in the study. Tumor samples were collected and processed for whole-slide imaging to analyze the tumor’s molecular and cellular heterogeneity. Seven biomarkers—GATA6, CLDN18, TFF1, MUC16, S100A2, KRT17, and PanBS—were selected for immunohistochemistry (IHC) staining on tissue sections. These markers were chosen due to their known roles in PDAC progression and differentiation. Digitized whole-slide images of both hematoxylin and eosin (H&E)-stained and IHC-stained sections were analyzed using unsupervised clustering to identify distinct tumor subgroups and assess intratumor heterogeneity.
Study Design
This was a retrospective, observational study that utilized tissue samples from patients who underwent surgery for resected PDAC. Whole-slide imaging, combined with unsupervised clustering, allowed for a systematic analysis of tumor characteristics and molecular expression patterns. The aim was to map out the different tumor clusters based on biomarker expression and evaluate their association with clinical outcomes such as survival and recurrence.
Results
The analysis of 120 PDAC tissue samples revealed significant molecular heterogeneity, with four distinct biomarker-driven subtypes identified. Each subtype demonstrated different prognostic implications, with MUC16-high and GATA6-high subtypes linked to poorer outcomes, while TFF1-high and S100A2-high subtypes were associated with better survival and reduced recurrence rates. Intratumor heterogeneity was also observed, reinforcing the complexity of PDAC and its potential implications for personalized treatment.
- A total of 120 PDAC tissue samples were analyzed, with biomarker expression patterns assessed using immunohistochemistry and quantitative PCR.
- Four distinct molecular subtypes of PDAC were identified, with each subtype exhibiting a unique biomarker profile: MUC16-high, GATA6-high, TFF1-high, and S100A2-high.
- The MUC16-high and GATA6-high subtypes were associated with a significantly higher risk of poor prognosis and recurrence.
- The TFF1-high and S100A2-high subtypes were linked to improved survival and lower recurrence rates, suggesting a more favorable outcome.
- Intratumor heterogeneity was observed, with some tumors displaying mixed patterns of biomarker expression, highlighting the complexity of PDAC.
- Statistical analysis confirmed the prognostic significance of these subtypes, with both MUC16 and GATA6 showing a strong correlation with poor overall survival (OS).
- Patients with the TFF1-high and S100A2-high subtypes demonstrated a longer median progression-free survival (PFS) compared to other subtypes.
- Unsupervised clustering of biomarker data was able to accurately predict patient outcomes, underscoring the potential for molecular profiling in guiding treatment strategies.
- Unsupervised clustering of biomarker data
The findings suggest that molecular profiling using a panel of biomarkers could be an effective tool in predicting patient outcomes
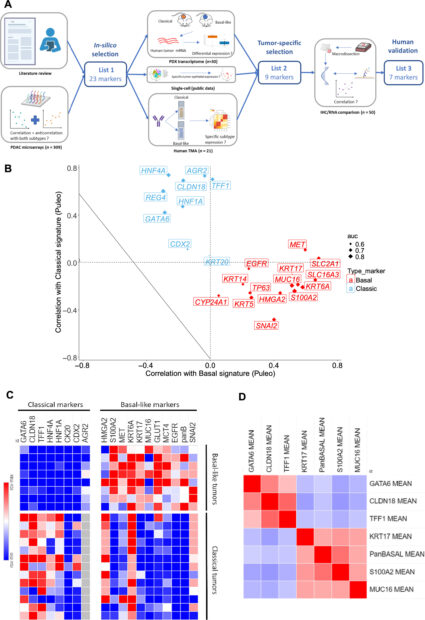
Figure 1 From Original Article: Marker selection to determine molecular subtype. (A) Multistep process to identify IHC marker surrogate of classical and basal-like subtypes. IHC, immunohistochemistry; PDAC, pancreatic ductal adenocarcinoma; PDX, patient-derived xenografts; TMA, tissue microarray. Created with Biorender.com. (B) Scatterplot showing correlation of gene expressions according to classical and basal signatures from Puleo et al (Cohort 1). Size of points is modulated by value of AUC predicting Purist signature. Basal markers are in red, classical markers in blue. (C) Heatmap representing IHC expression of tested markers in set of 13 classical and eight basal-like pancreatic ductal adenocarcinomas from Cohort 1. H-score: 0 (blue) to 300 (red). (D) Correlation matrix of the seven validated IHC markers in 50 human PDACs from Cohort 2 (red = strong positive correlation, blue = strong negative correlation).
Key Findings
This study identified several important insights into the molecular heterogeneity of PDAC. The analysis revealed distinct subtypes of PDAC based on biomarker expression patterns, with varying prognostic implications. Tumors exhibiting higher expression of MUC16 and GATA6 were associated with poorer outcomes, while those with elevated TFF1 and S100A2 expression showed a more favorable prognosis. The high degree of intratumor heterogeneity emphasizes the potential for personalized treatment strategies tailored to molecular subtypes.
- The study identified distinct molecular subtypes within PDAC tumors, associated with variations in biomarker expression, which had significant prognostic value.
- There was a high level of intratumor heterogeneity, with mixed phenotypic expressions observed in some tumors, further complicating treatment strategies.
- Tumors expressing high levels of MUC16 and GATA6 were linked to poorer survival rates, while higher expression of TFF1 and S100A2 indicated a more favorable prognosis.
- Unsupervised clustering provided valuable insights into the complexity of PDAC, highlighting the need for tailored therapeutic strategies based on molecular profiling.
Key Takeaway Messages
The findings emphasize the complexity of PDAC and the critical role of intratumor heterogeneity in influencing clinical outcomes. A multi-marker approach to tumor characterization provides a more accurate depiction of the disease and could help guide more personalized treatment strategies.
- PDAC tumors exhibit distinct molecular subtypes with significant variability in biomarker expression.
- A high degree of intratumor heterogeneity complicates treatment decisions, as tumors within a single patient may exhibit differing phenotypes.
- Tumors with elevated MUC16 and GATA6 expression were linked to poorer prognosis, whereas high TFF1 and S100A2 expression indicated better clinical outcomes.
- Unsupervised clustering provided a valuable tool for identifying PDAC subtypes, suggesting potential for personalized treatment strategies.
Conclusion
This study underscores the importance of characterizing PDAC tumors beyond standard histopathological assessment by incorporating molecular markers to assess intratumor heterogeneity. By utilizing a multi-marker profiling approach, clinicians can better understand the molecular complexity of PDAC, which could lead to more effective personalized treatment plans. The results suggest that refining tumor classification and tailoring therapy based on individual molecular profiles could improve outcomes in patients with PDAC.
You Can Read the Full Article Here
Summary by Sona Karamyan, MD


