Advanced hepatocellular carcinoma (advanced HCC) remains a leading cause of cancer-related mortality, with systemic therapy playing a crucial role in disease management. The treatment paradigm has rapidly evolved, shifting from tyrosine kinase inhibitors (TKIs) like sorafenib to immune checkpoint inhibitor (ICI)-based combinations. Landmark phase III trials, including IMbrave150, HIMALAYA, CARES-310, and CheckMate 9DW, have established atezolizumab–bevacizumab, durvalumab–tremelimumab, camrelizumab–rivoceranib, and nivolumab–ipilimumab as preferred first-line options. This article, published in ESMO Gastrointestinal Oncology 2025, explores the clinical impact of these regimens, highlighting efficacy, toxicity, and future directions in optimizing treatment for advanced HCC.
Authors: A. Martirena, P. Lombardi , D.J. Pinato , L. Rimassa , A. Lamarca
Background
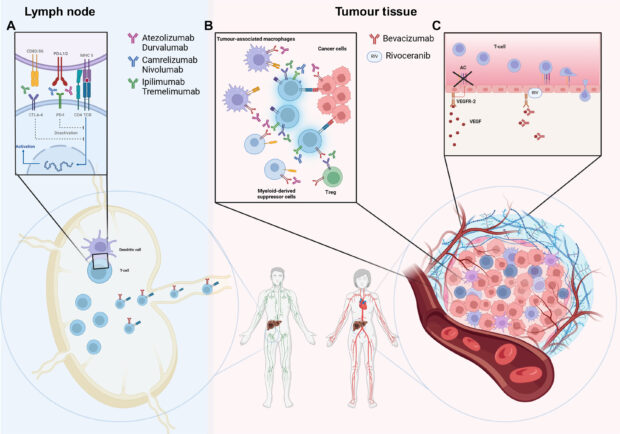
Hepatocellular carcinoma (HCC) is one of the leading causes of cancer-related mortality worldwide, primarily due to late-stage diagnosis and limited curative options. For over a decade, tyrosine kinase inhibitors (TKIs) such as sorafenib and lenvatinib remained the cornerstone of systemic therapy. However, the landscape has undergone a dramatic transformation with the advent of immune checkpoint inhibitors (ICIs), particularly combinations targeting PD-1, PD-L1, CTLA-4, and VEGF pathways.
Despite these advancements, no validated biomarkers exist to guide treatment selection, making clinical judgment essential in choosing optimal therapy. The choice among available regimens depends on efficacy, safety, and patient-specific factors, necessitating a nuanced understanding of pivotal trials shaping current practice.
Pivotal Clinical Trials in First-Line Therapy
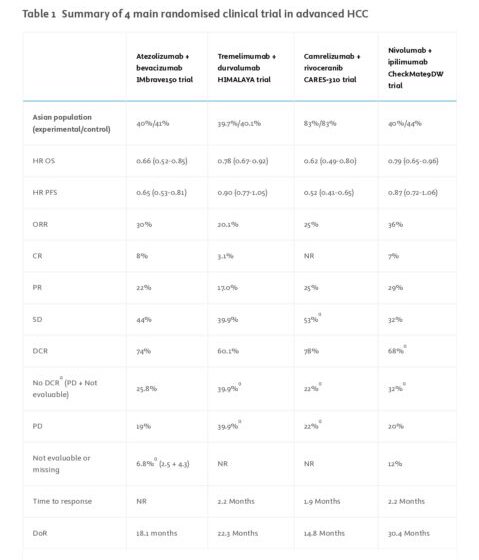
Four recent phase III randomized controlled trials have established immune-based combinations as the preferred first-line therapy for advanced HCC, significantly improving survival compared to TKIs.
1.IMbrave150 (2020): Atezolizumab + bevacizumab vs. sorafenib
2.HIMALAYA (2022): Durvalumab + tremelimumab vs. sorafenib
3.CARES-310 (2023): Camrelizumab + rivoceranib vs. sorafenib (predominantly Asian cohort)
4.CheckMate 9DW (2024): Nivolumab + ipilimumab vs. sorafenib/lenvatinib
IMbrave150: Atezolizumab + Bevacizumab
- Study Design: Phase III, open-label trial comparing atezolizumab (anti-PD-L1) + bevacizumab (anti-VEGF) vs. sorafenib.
- Results:
- Overall survival (OS): HR 0.66 (95% CI: 0.52–0.85), median OS 19.2 months.
- Progression-free survival (PFS): 6.8 months vs. 4.3 months with sorafenib.
- Objective response rate (ORR): 30%.
- Toxicity: Increased risk of hypertension (29%) and bleeding (7%), requiring caution in patients with varices.
Atezolizumab–bevacizumab remains the preferred first-line option when antiangiogenic therapy is feasible.
HIMALAYA: Durvalumab + Tremelimumab (STRIDE regimen)
- Study Design: Phase III, open-label trial comparing durvalumab (anti-PD-L1) + single priming dose of tremelimumab (anti-CTLA-4) vs. sorafenib.
- Key Findings:
- OS: HR 0.78 (95% CI: 0.67–0.92), median OS 16.4 months.
- Long-term survival benefit: 30.7% alive at 3 years, 19.6% at 5 years.
- ORR: 20%.
- Toxicity: Lowest rate of grade 3–4 adverse events (25.8%) among ICI regimens.
Clinical Implication: STRIDE is a strong alternative when VEGF inhibitors are contraindicated, offering durable long-term survival with favorable safety.
CARES-310: Camrelizumab + Rivoceranib
- Study Design: Predominantly Asian phase III trial comparing camrelizumab (anti-PD-1) + rivoceranib (VEGFR-2 inhibitor) vs. sorafenib.
- Results :
- OS: HR 0.62 (95% CI: 0.49–0.80), median OS 22.1 months.
- PFS: HR 0.58, median PFS 5.6 months.
- ORR: 25%.
- Toxicity: Highest grade 3–4 adverse event rate (88%), particularly hypertension (38%).
Promising OS benefit but high toxicity limits widespread adoption outside Asia.
CheckMate 9DW: Nivolumab + Ipilimumab
- Study Design: Phase III trial comparing nivolumab (anti-PD-1) + ipilimumab (anti-CTLA-4) vs. sorafenib/lenvatinib.
- Key Findings:
- OS: HR 0.79 (95% CI: 0.65–0.96).
- ORR: Highest among all regimens (36%), with 13% achieving complete response.
- Toxicity: 30% of patients required high-dose steroids for immune-related adverse events.
High ORR makes nivolumab–ipilimumab an option for patients requiring rapid tumor shrinkage, but toxicity necessitates close monitoring.
Key Takeaways
- TKIs remain an option only if immunotherapy is contraindicated.
- Atezolizumab–bevacizumab is the preferred first-line option due to efficacy and favorable safety profile, barring contraindications to antiangiogenic therapy.
- Durvalumab–tremelimumab (STRIDE regimen) is a viable alternative if antiangiogenic agents are unsuitable, offering long-term survival benefits and a favorable toxicity profile.
- Nivolumab–ipilimumab may be considered for select patients where achieving tumor response is critical (e.g., potentially resectable disease).
- Future directions include triplet regimens, bispecific antibodies, and integration with locoregional therapies. Biomarkers remain an urgent unmet need.
Conclusion
The systemic treatment landscape for advanced HCC has shifted from TKIs to immune-based combinations, significantly improving survival. Four regimens—atezolizumab–bevacizumab, durvalumab–tremelimumab, camrelizumab–rivoceranib, and nivolumab–ipilimumab—are now available as first-line options. Treatment decisions must consider efficacy, toxicity, and patient-specific factors due to the absence of predictive biomarkers. Ongoing research into novel triplet therapies, bispecific antibodies, and biomarker-driven strategies will further refine therapeutic approaches, ultimately personalizing treatment for patients with advanced HCC.
Summary by Sona Karamyan, MD