Marco Donia, Professor at the University of Copenhagen and Research Group Leader (TIL group) at CCIT – Center for Cancer Immune Therapy, shared a post on LinkedIn:
“Neoantigen-Specific TILs in Gastrointestinal Cancers (Nature Medicine, Frank J Lowery III, Stephanie Goff et al., 2025)
Adoptive cell therapy with tumor-infiltrating lymphocytes (TILs) has reshaped melanoma treatment, but its efficacy in gastrointestinal (GI) cancers has been limited – until now.
This new phase 2 study evaluated 91 patients with treatment-refractory, mismatch repair proficient (pMMR) metastatic GI cancers, treated with bulk TILs (as we do in melanoma) or Selected TILs (neo-antigen selected)
Key Findings:
Response Rates:
- 0/18 responses in the bulk TIL arm.
- 3/39 (7.7%) partial responses in the SEL-TIL arm.
- 8/34 (23.5%) partial responses in the SEL-TIL + anti-PD-1 arm (reflecting a boost when targeting PD-1 in tumor-reactive TILs, more.
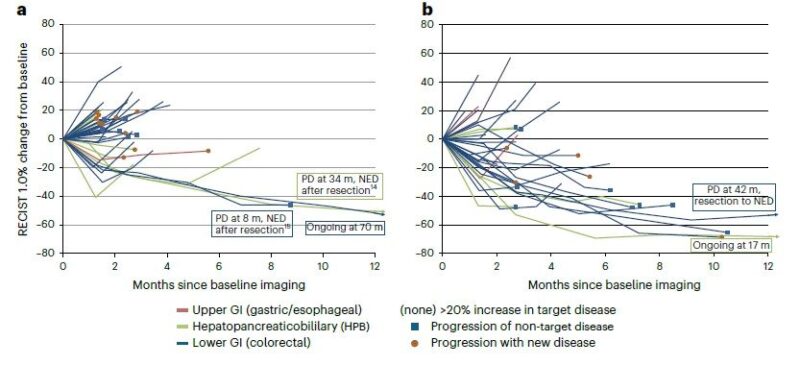
Durability of Response:
- SEL-TIL: 8, 24, and 70+ months.
- SEL-TIL + anti-PD-1: 4, 7, 7, 8, 10, 11, 17+, and 42 months.
Feasibility and Attrition:
- 168 patients screened for neoantigen reactivity, 95 (57%) had successful neoantigen-reactive TILs (median time to screening completion: 2.6 months)
- 73 patients (43%) proceeded to treatment (disease progression, poor TIL growth, or declining clinical status)
Correlates of Response:
- More infused neoantigen-specific CD4+ T cells (median 2.4 × 10¹⁰ vs 0.4 × 10¹⁰).
- Greater number of unique tumor neoantigen targets (median 3 vs 2).
- Inflammatory gene signatures in responding tumors.
Safety:
- 100% experienced grade ≥3 hematologic toxicity, as expected
- One treatment-related death (adenoviral hepatitis) occurred.
Take-Home:
– Personalized TIL therapy targeting patient-specific neoantigens can achieve meaningful and durable responses in pMMR GI cancers, previously considered non-responsive to immunotherapy.
– PD-1 blockade adds clinical benefit (moving forward in expansion phase).
– Some responses were durable, yet appear less durable than in melanoma.
– Manufacturing success rate of 57%: encouraging, and high dropout (manufacturing time >2 months) calls for streamlined production timelines.
Clinical Implications:
– Nandmark step in extending TIL therapy to common epithelial cancers.
– Highlights the importance of individualized immunotherapy and necessity to address logistical hurdles in cell therapy development.
– Ongoing trial: NCT01174121
Great study from colleagues at the National Cancer Institute (NCI).”
Neoantigen-specific tumor-infiltrating lymphocytes in gastrointestinal cancers: a phase 2 trial
Authors: Frank J. Lowery, Steven A. Rosenberg et al.

Further Reading:
Melanoma (Skin Cancer): Symptoms, Causes, Stages, Diagnosis and Treatment


