
Updates on BOT/BAL from ESMOGI24
ESMO GI 2024 is held in Munich, Germany, and online from June 26-29. Organized by the European Society for Medical Oncology, the event presents new data in GI oncology, alongside educational sessions and networking opportunities. The congress aims to highlight recent advancements in GI cancer treatment and ensure their clinical implementation. It also provides global visibility for original clinical and translational research.
Agenus Inc. announced significant tumor reductions in MSS colon cancer patients treated with botensilimab/balstilimab from the NEST clinical trial. The results were presented at the ESMO GI 2024 congress.
Agenus shared on their X page:
“New data from the NEST clinical trial was presented at European Society for Medical Oncology’s ESMOGI24 – results demonstrated significant tumor reductions in MSS colon cancer patients treated with botensilimab/balstilimab in the neoadjuvant setting.”

Cathy Eng, Co-Director of GI Oncology and Co-Leader of the Gastrointestinal Cancer Research Program at the Vanderbilt-Ingram Cancer Center, shared on X/Twitter:
“Bot/Bal immunotherapy in colon cancer NEST 1/2.
Agenus with very promising data in early MSI-s/MSI-H colon cancer!”
Arndt Vogel, Managing Senior Consultant and a Professor in the Department of Gastroenterology, Hepatology, and Endocrinology at Hannover Medical School, shared on X:
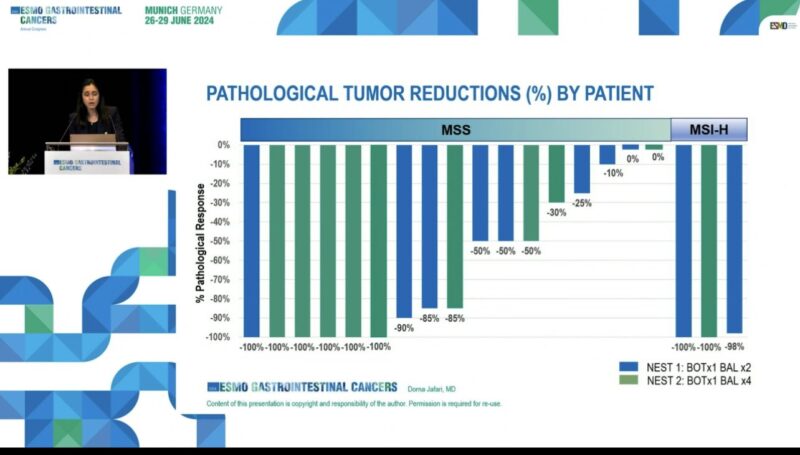
“Neoadjuvant botensilimab + balstilimab in MSI and MSS CRC at ESMOGI24.
- NEST clinical trial, 20 MSS, 3 MSI,
- pMSS: 50% cPCR rate,
- Really active in MSI and MSS CRC.”
Vittorio Studiale, Medical Oncology Resident at University Hospital of Pisa, shared on X:
“Impressive pathological response rate (71%, pCR 35%) with neoadjuvant BOT/BAL in MSS colorectal cancer from the update of NEST-1 trial.”
Paolo Ciracì, Oncology Resident at University Hospital of Pisa, shared on X:
“Updated results from the NEST-1 trial at ESMOGI24.
- 6/9 MSS pts: ≥50% major pat response (2/9 pCR),
- Median TMB in MSS was 3.7 Mut/Mb,
- Increased T cell infiltration, elevated CD8+/Treg and higher proportion of activated macrophages in post-resection specimens.”
ESMO (European Society for Medical Oncology) shared on LinkedIn:
“Data from two presentations at ESMOGI24 highlight the clinical utility of liquid biopsies detecting circulating tumor DNA (ctDNA) in guiding treatment for patients with Metastatic Colorectal Cancer (mCRC) and providing prognostic information post-surgery for patients with stage 3 colon cancer.
To learn more about this topic, read the analysis from Pashtoon Kasi in the latest ESMO Daily Reporter article.”

-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
