Mark Yarchoan, Associate Professor at Johns Hopkins Hospital, shared on X:
“Multicenter P1/2 trial of a personalized neoantigen vaccine + anti-PD1 (pembrolizumab) in 2L HCC presented at AACR24 and published in Nature Medicine.
ORR 31%, increased historical control of pembrolizumab monotherapy (~17%)
Details below.
36 patients in US (Mount Sinai NYC, Johns Hopkins Kimmel Cancer Center) and The University of Auckland.
- Patients enrolled on 1L TKI treatments (sora or lenva),
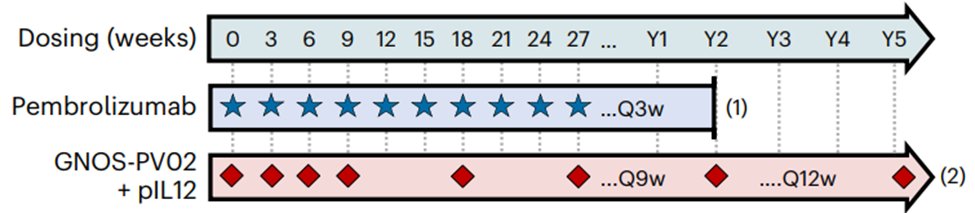
- DNA vaccine with pIL12 adj targeted up to 40 neos.
ORR 30.6% (11 of 36 patients), 8.3% complete responses
- Superiority versus the prespecified historical control (pembrolizumab monotherapy ORR ~17% in HCC),
- Randomized trials needed to confirm.
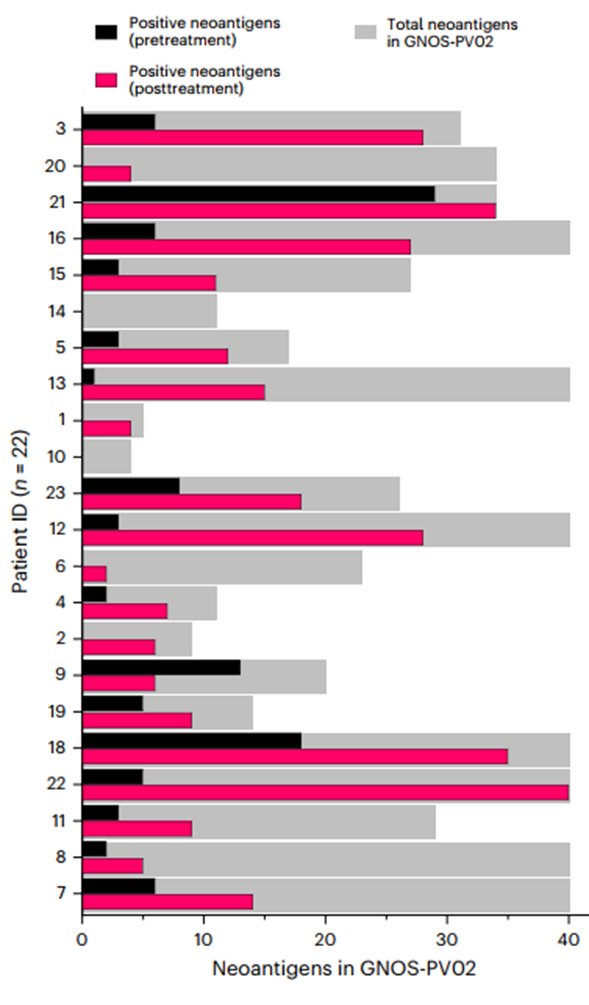
- New or increased neoantigen-specific T cell responses detected in 19/22 patients in peripheral blood
- Better clinical outcomes for patients with 1) increased neos included in vaccine, and 2) increased strength of neoantigen-specific response.
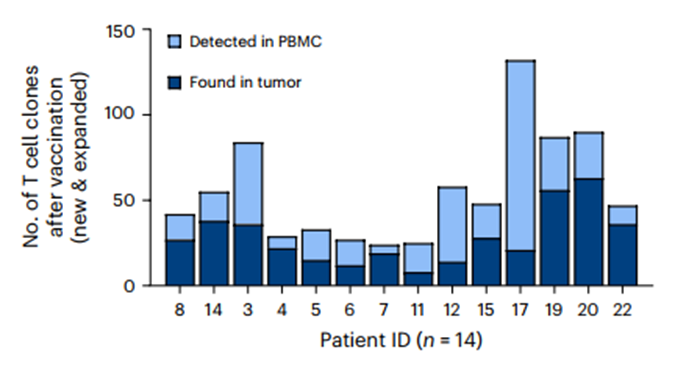
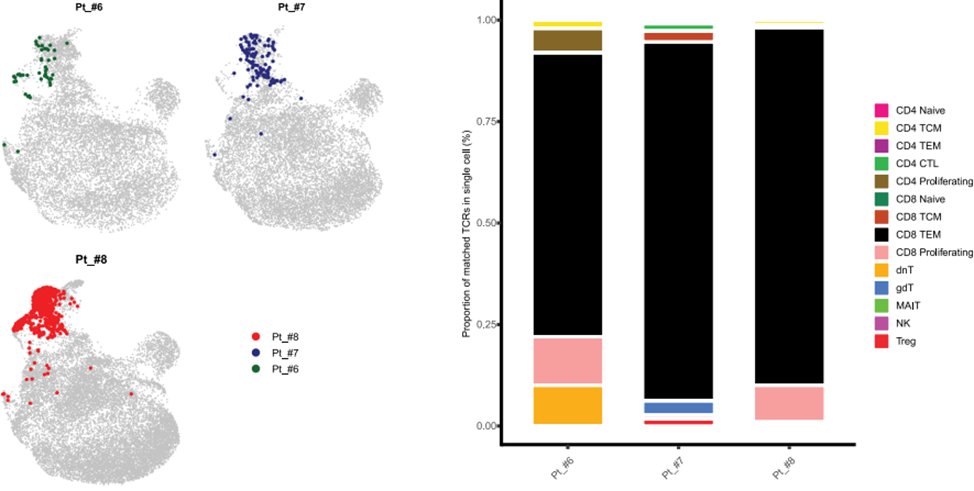
- Significant T cell clonal expansion in 14/14 (100%) patients in peripheral blood and tumor
- 3 expanded TCR clones were cloned and demonstrated specificity for vaccine-encoded antigens
- Expanded TCRs corresponded to highly activated CD8+ and to a lesser degree CD4 effector T cells.
- Builds on recent success with melanoma and panc vax in micromet setting; efficacy may extend into advanced disease,
- HCC an ideal tumor because T cell priming is impaired in liver (and overcome by vax) – maybe also explain anti-CTLA4 efficacy in HCC.
Congratulations to all authors, Ed Gane, Tom Marron, Daniel Shu,Elana Fertig, Elizabeth Jaffee, Luciane Kagohara, Geneos Therapeutics, and thank you Niranjan Sardesai for the opportunity to join this study even when I was a very junior faculty.”
Proceed to the article.
Source: Mark Yarchoan/X