Vincent Rajkumar, Professor of Medicine at the Mayo Clinic, shared a post on X:
“Summary of drug approvals in relapsed myeloma. 19 new drugs approved over 20 years.
Bookmark it!
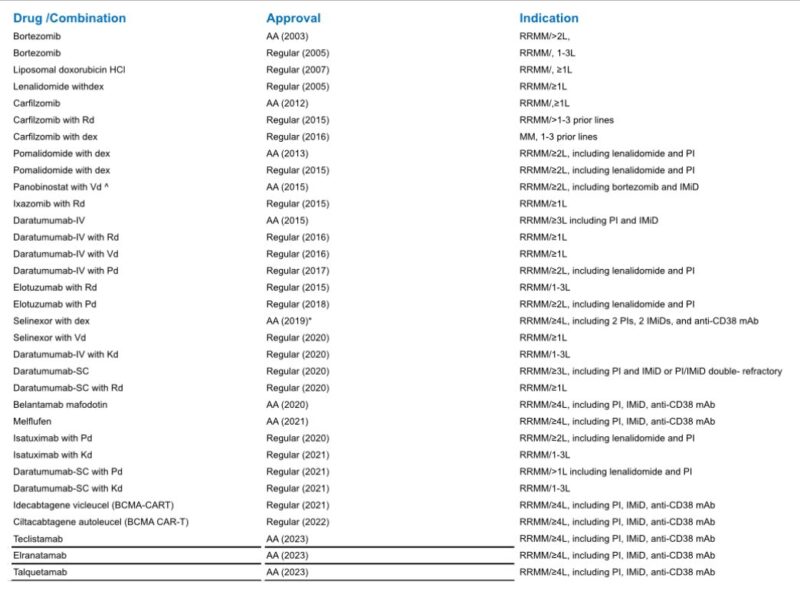
AA indicates accelerated approval.
Using AA ahead of regular approval, drugs have become available for patients 2-3 years earlier on average than if we had waited for regular approval through phase III data.
Of 11 myeloma drugs with AA, 3 drugs had issues:
Panobinostat – confirmatory trial not done
Melflufen – confirmatory trial did not confirm expected benefit
Belantamab – 1st confirmatory trial negative, but 2nd shows marked benefit so will likely be back on market.
Having accelerated approval using single arm trials there is always a trade off between lives saved by having effective drugs approved 2-3 years early versus lives lost by approval of an ineffective or harmful drug.
This is discussed in this thread.
In myeloma, far more lives have been prolonged by having the accelerated approval pathway available, and potential lives lost from approval of ineffective drugs is minimal if any:
Very few used melflufen. Belantamab is effective and will be back. Panobinostat is the unknown.
In myeloma, most accelerated approvals turned out to be the right call. This may be unique; not generalizable.
Myeloma is unique in that we have very reliable markers of tumor burden (M protein/FLC) we can measure monthly, evaluated response, and adjust drugs, dosage, duration.
At the Myeloma Society FDA meeting we emphasized that current approach to myeloma drug approvals has served us well & accelerated approval based on single arm trials should continue.”


