David B. Solit, Director, Center for Molecular Oncology at Memorial Sloan Kettering Cancer Center, posted the following on X:
“It has been a year since my last update on the prospective MSK-IMPACT/ACCESS sequencing cohort, now >120,000 tumor/cfDNA samples analyzed. Thanks to the patients, docs, nurses, techs, data scientists who have contributed. So where are we going next:
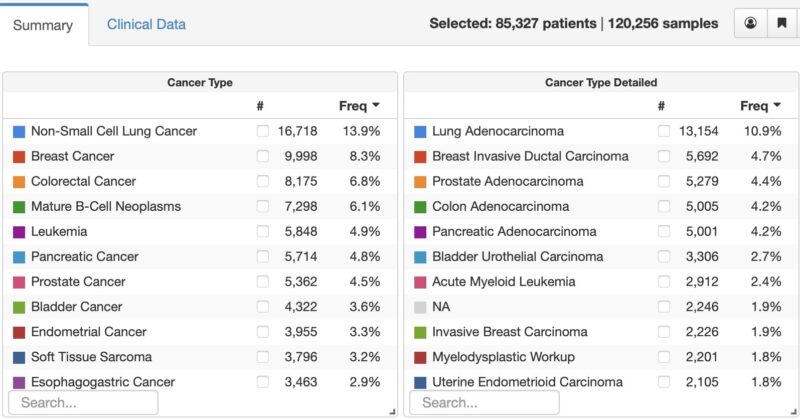
This is an increase in 20,227 tumor/cfDNA samples over the past year, but only 11,736 new patients enrolled. This reflects 1) the need to sequence more than one sample for some patients – both tumor + cfDNA, repeat at time of recurrence, and 2) New second cancers for some pts.
Why 2,246 patients with NA (not available) as their diagnosis. This reflects the increasing use of tumor genomic profiling for diagnostic purposes versus as a guide to treatment, in particular in hematologic cancers. So what’s next:
Broader sequencing (whole genome): The main hurdles are 1) high cost, 2) generating quality WGS on FFPE tumors is still a challenge. I do not expect much progress on this front over the next year. Need sequencing cost to decline to make clinical WGS a reality at scale.
I do expect cfDNA panels will increase in size given small incremental declines in sequencing costs. This will make cfDNA even more impactful for both diagnostic and therapeutic decision making.
Complementary assays – RNA sequencing. This should be the highest priority as it would add the greatest value to the targeted DNA sequencing panels already widely available. Issue will be re-imbursement as it will supplement and not replace DNA sequencing.
For clinical RNA sequencing to become reality, we need to demonstrate clinical impact. Optimistic that RNAseq will prove more useful for 1) identifying fusions, and 2) guiding immunotherapy and ADCs than DNA sequencing has been to date.
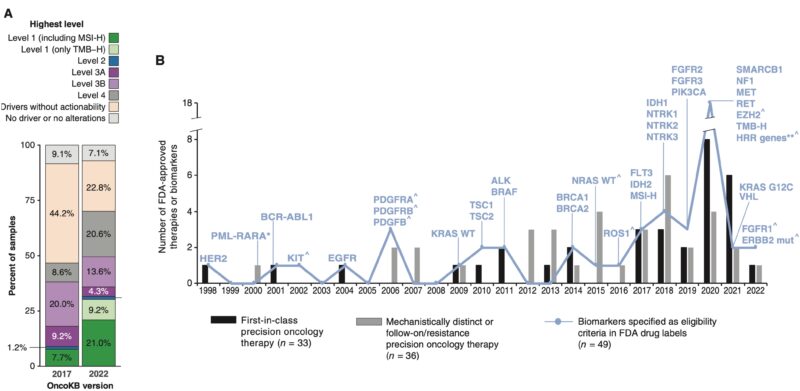
But, the biggest unmet need is more/better drugs. I would refer people to the recent OncoKB paper which characterized the fraction of patients with standard care biomarkers of response detected by tumor NGS (31.6% up from 8.9% in 2017).”
Source: David B. Solit/X
