Replimune’s RP1 for Advanced Melanoma Has Received BLA Acceptance and Priority Review
Replimune Group has announced that the FDA has accepted its Biologics License Application for RP1 in combination with nivolumab for the treatment of advanced melanoma.
The FDA granted the BLA Priority Review, with a PDUFA action date of July 22, 2025. This acceptance marks a significant milestone for Replimune in advancing its novel oncolytic immunotherapy platform.
The BLA submission is supported by the primary analysis data from the IGNYTE trial, which evaluated RP1 combined with nivolumab in patients with advanced melanoma who had failed prior anti-PD-1 therapy. The results from this study provided the clinical evidence necessary to move the application forward. The FDA has indicated that, at this time, there are no identified review issues, and they are not planning to hold an advisory committee meeting related to this application.
Replimune is conducting a confirmatory Phase 3 trial, IGNYTE-3, which is evaluating the combination therapy in a broader patient population. This trial is planned to include over 100 clinical sites globally, further strengthening the clinical development program for RP1 in the treatment of advanced melanoma.
About The IGNYTE-3 trial
The IGNYTE-3 trial is a randomized, controlled, multicenter, open-label Phase 3 clinical study designed to evaluate the efficacy and safety of vusolimogene oderparepvec (VO) in combination with nivolumab compared to Physician’s Choice treatment for patients with unresectable Stage IIIb-IV cutaneous melanoma. These patients must have experienced disease progression after receiving treatment with an anti-PD-1 and an anti-CTLA-4 containing regimen, either administered as a combination regimen or sequentially. Additionally, patients who are not candidates for treatment with an anti-CTLA-4 therapy are also eligible for the study.
The official title of the trial is “Randomized, Phase 3 Clinical Study Comparing Vusolimogene Oderparepvec in Combination With Nivolumab Vs Treatment of Physician’s Choice in Patients With Advanced Melanoma That Progressed on Anti-PD-1 and Anti-CTLA-4 Containing Treatment.” This trial aims to provide critical data on whether the combination of VO and nivolumab offers a better clinical outcome for these patients compared to the standard treatment options available, providing further evidence for the potential use of this novel combination therapy in treating advanced melanoma.
About RP1 (vusolimogene oderparepvec)
RP1 (vusolimogene oderparepvec) is Replimune’s lead product candidate, designed as an innovative oncolytic immunotherapy to treat cancer, particularly advanced melanoma. It is based on a proprietary strain of herpes simplex virus (HSV) that has been genetically engineered and armed with a fusogenic protein (GALV-GP R-) and GM-CSF. This unique combination enhances the virus’s ability to selectively infect and destroy tumor cells while minimizing damage to healthy tissue. The fusogenic protein facilitates the fusion of the virus with tumor cell membranes, promoting viral spread and amplified tumor cell death. GM-CSF, a cytokine, is included to boost the immune response by stimulating the recruitment and activation of immune cells, thereby enhancing the body’s ability to recognize and attack tumor cells. The dual mechanism aims not only to directly kill tumor cells but also to trigger a systemic immune response against the tumor, potentially leading to long-lasting immunity. By targeting and killing cancer cells, RP1 seeks to offer a powerful therapeutic strategy for patients with hard-to-treat cancers, such as melanoma, by leveraging both viral oncolysis and immune system activation to improve clinical outcomes.
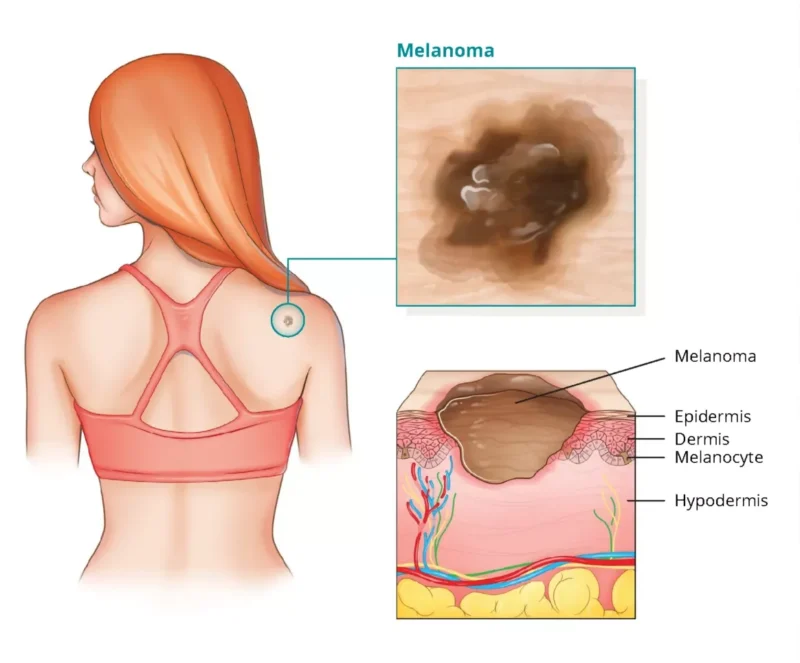
About Melanoma
Melanoma is the fifth most common cancer, with approximately 100,000 new cases and 8,000 deaths projected in the U.S. in 2024. The standard of care for advanced melanoma often includes immune checkpoint blockade therapies, such as those targeting PD-1 or CTLA-4 pathways, which work by enhancing the body’s immune response against tumor cells. However, around half of the patients do not respond to these treatments, and many others will experience disease progression after an initial period of effectiveness. This highlights a significant gap in treatment options, as after immune checkpoint blockade therapy, limited therapeutic alternatives are available. Unfortunately, there is currently no standard of care for patients who have failed these frontline immunotherapies, underscoring the need for innovative treatments that can address this unmet need and offer improved outcomes for these patients.
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
