Saarang Deshpande shared a post on X:
“Thread on frontline caplacizumab and plasma exchange in TTP. With advancing care for immune thrombotic thrombocytopenic purpura (iTTP), let’s discuss frontline caplacizumab (capla) and the future for therapeutic plasma exchange (TPE).
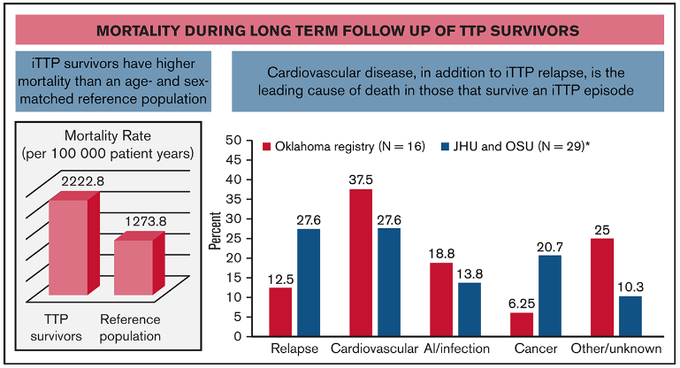
The early days of acute iTTP are of paramount importance: it appears to be the time when a majority of the morbidity (stroke, MI, etc) and mortality occurs Prompt dx and tx prevents early (functionally-limiting?) end-organ damage for a disease with prolonging survivorship.
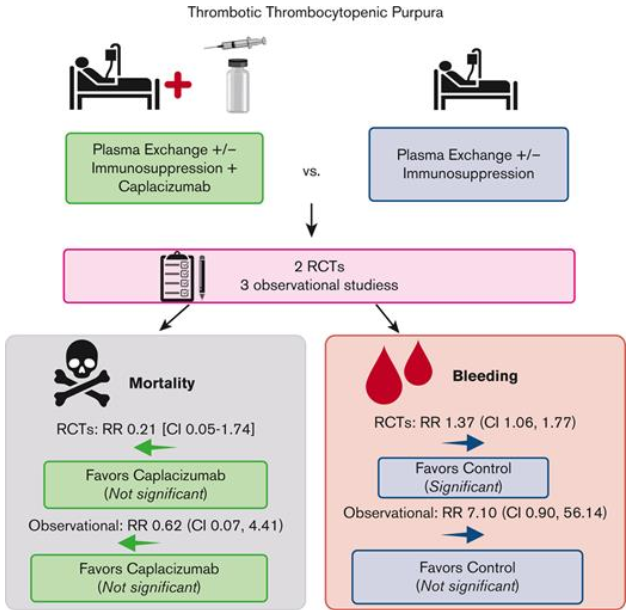
Caplacizumab, a nanobody inhibiting VWF and platelet GpIb interaction, was approved for iTTP in 2018 based on the TITAN and HERCULES trials, which reached primary outcomes of time to plt recovery However, use of frontline capla is debated.
Caplacizumab Treatment for Acquired Thrombotic Thrombocytopenic Purpura
Authors: Marie Scully, et al.

Meta-analysis showed non-sig mortality, but trials weren’t powered for such an outcome…unfortunately And it doesn’t address iTTP’s underlying cause: autoantibodies to ADAMTS13. Rather, it places a band-aid on uncontrolled microvascular thrombosis.
Authors: Mia DjulbegovicJiayi TongAlice XuJoanna YangYong ChenAdam Cuker andAllyson M. Pishko.
With mortality ~20% even recently, most before tx or within 10 days of dx, upfront capla to mitigate end-organ injury can make sense The benefits? refractory TTP and exacerbation, time to response, time in hospital / on TPE… But unclear longer-term clinical benefit.
Now that we’ve described the conditional ISTH recommendation of frontline capla, where does that leave TPE? TPE with plasma replacement is a cornerstone of TTP mgmt: started daily on presumed TTP dx until cessation of end-organ dysfxn & hemolysis and plt recovery.
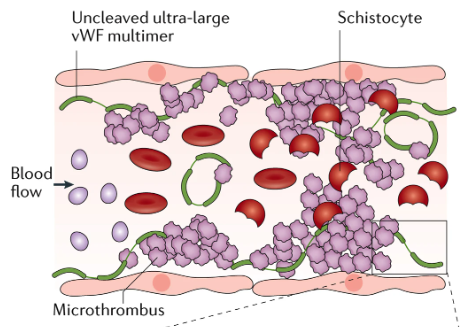
TPE replaces ADAMTS13, removes ADAMTS13 inhibitors, and removes residual ultra-large VWF multimers. So why reconsider the role of frontline TPE? TPE requires CVC placement (usually) and ICU-level care & has logistical difficulty, complications, and lots of plasma exposure.
Historically, ~25% of TPE for TTP had major complications (bacteremia, catheter thrombosis, procedural), though that’s likely far lower now with shorter TPE duration and POCUS-guided placement. Now for the good stuff… a large series describing TPE-free reigmens for iTTP.
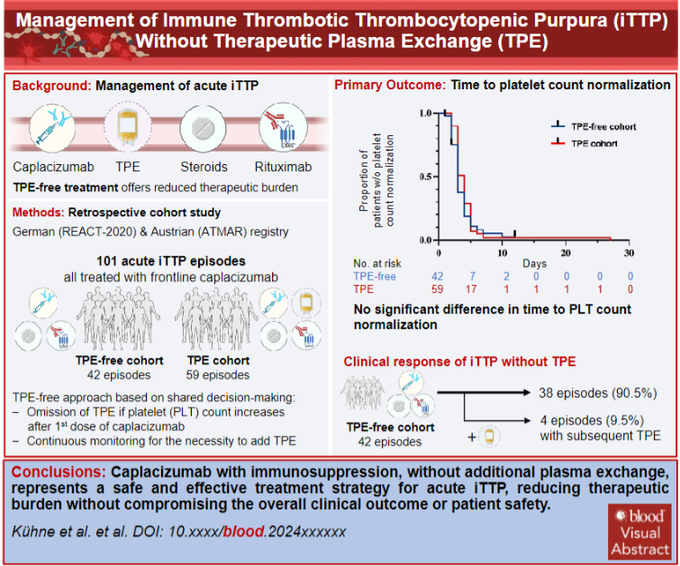
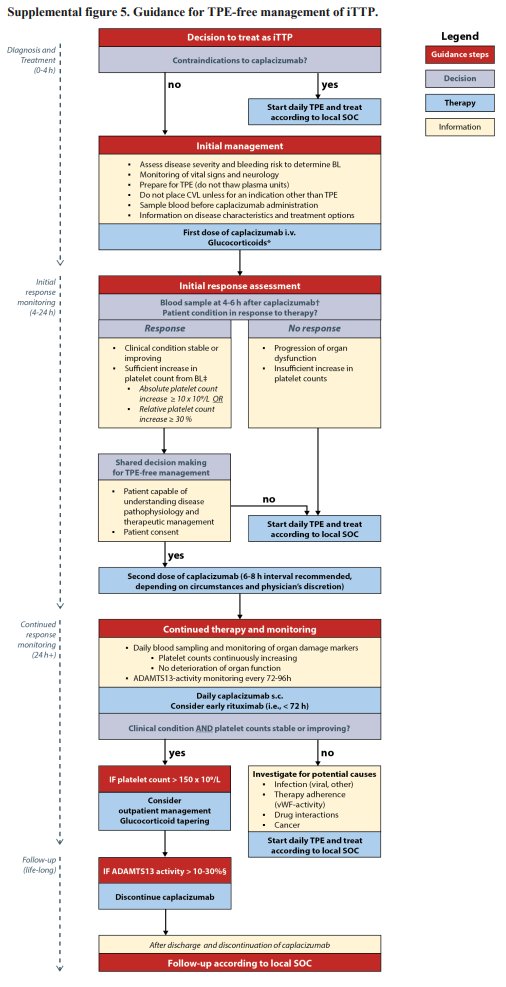
In the post-capla era, an Austrian/German team studied a TPE-free approach to acute TTP in a retrospective cohort. They started with capla and pursued TPE-free mgmt if plt count and stable sx / end-organ injury at short interval assessment.
Management of Immune Thrombotic Thrombocytopenic Purpura without Therapeutic Plasma Exchange
Authors: Lucas Kühne, et al.
Primary outcome of time to plt count normalization was similar between TPE (4 days) and TPE-free (3 days) approaches Similar secondary outcomes included (TPE vs TPE-free)
Clinical response (97 vs 90%)
Exacerbations (15 vs 5%)
Refractory TTP (1 pt vs none)
Bleeding.
ADAMTS13 recovery >20% was shorter in the TPE-free cohort (25 vs 37 days) as were in-hospital days. 4 pts (out of 42 episodes) in the TPE-free cohort ultimately got TPE, starting ~1.5 days after capla, primarily due to poor plt response. None were refractory.
The authors sum it up nicely with a proposed algorithm for initiating a TPE-free approach to acute TTP. Much of this rests on shared decision-making and very close monitoring of initial response to capla with the institutional support to pivot to TPE at a moment’s notice.
There’s precedent for TPE-free approaches to acute iTTP A NEJM case report managed iTTP with capla in a Jehovah’s Witness who declined TPE:
Caplacizumab Therapy without Plasma Exchange for Acquired Thrombotic Thrombocytopenic Purpura
This has been described previously w/o capla as well:
Authors: James N. George, Steven A. Sandler and Joanna Stankiewicz.

A recent exceptional NEJM case report described the use of rADAMTS13 (Takeda) in a case of severe refractory TTP in which capla was initially deferred due to vaginal bleeding and, later, giving capla led to significant GIB.
Recombinant ADAMTS13 for Immune Thrombotic Thrombocytopenic Purpura
Authors: Pavan K. Bendapudi, Brody H. Foy, Sarah B. Mueller, Jun Liu, Louis M. Feingold, Kristen E. Burke, Wendy Cruz, Maria Y. Chen, Emily S. Lau, Rachel L. Goldberg, Ishan Tatake, Shelby C. Wilkinson, Brian J. Carney, James R. Stone, Doyun Park, Alzira R. Avelino, Sajjad Hassan, Chester Andrzejewski, Kristen N. Ruby, Kenneth D. Friedman, Patricia A.R. Brunker, Rebecca K. Leaf, John Higgins, Walter H. Dzik, Jonathan A. Stefely, and Robert S. Makar.

Phase 2 SOAR-HI (NCT03922308) trial showed rADAMTS13 in combination with TPE led to increased ADAMTS13 exposure with similar AEs across arms…so there’s proof of concept here.
Authors: Marie Scully, Jovanna Baptista, Indranil Bhattacharya, Spero Cataland, Paul Coppo, Loredana Cuccia, Tina Dutt, Shih-Han Susan Huang, Cristina Pascual Izquierdo, María Eva Mingot-Castellano, Aric Parnes, Katerina Pavenski, Kavitha Rajavel, Isidro Jarque, Linda T. Wang, Miguel Fernández Zarzoso, Andy Zhu and Björn Mellgård.
Some ongoing trials assessing the role of TPE in acute iTTP:
MAYARI (NCT05468320): Phase 3 trial for capla w/o firstline TPE
NCT05714969: Phase 2b trial for TAK-755 (rADAMTS13) with little to no TPE
PEX-FREE (NCT06291025): Capla + plasma infusion w/o TPE.
The cost-(in)effectiveness of frontline capla is important to consider too!
Projected ICER: 1.5 mil even after ‘stacking the deck’ in favor of capla
Capla is 1 of 6 FDA-approved meds denied reimbursement by 2+ developed countries b/w 2017-20.
Cost effectiveness of caplacizumab in acquired thrombotic thrombocytopenic purpura
Authors: George GoshuaPranay SinhaJeanne E. HendricksonChristopher TormeyPavan K. Bendapudi and Alfred Ian Lee.
Strict patient selection (severe TTP with neuro/cardiac damage, long inpt stay w/ TPE) and TPE-free approaches may soften the financial blow of capla, but it seems unlikely to become cost-effective regardless.
Big takeaways:
- Acute iTTP is most morbid and mortal early, so urgent dx is imperative. With data maturity, could we see longer-term effects of frontline caplacizumab blunting end-organ injury in early TTP? Neurocognitive and cardiovascular disease outcomes are two key endpoints to assess.
- Removing anything from frontline iTTP treatment feels precarious, but foregoing TPE in those with initial response to capla may be a forthcoming paradigm shift. How can we deploy this? What initial features are more likely to respond to TPE-free regimens?
- In the next few years, we’ll see data for rADAMTS13 in acute iTTP, which should decrease the concentration of ultra-large vWF in acute iTTP. Will it protect against end-organ injury? Will this be cost-effective?
- As a learner, I’m interested in practice patterns for frontline capla – all or only severe cases? Upfront or only refractory? Ever initiate capla in cases with very high suspicion w/o ADAMTS13 activity confirmed?”
Source: Saarang Deshpande/X
Saarang Deshpande is a Resident Physician at Penn Medicine, University of Pennsylvania Health System. He previously worked as a Research Assistant at Cornell University and completed internships at the American Psychiatric Association, VCU Health, and Johns Hopkins School of Medicine.