If you have been diagnosed with extensive-stage small cell lung cancer (ES-SCLC) and have already undergone platinum-based chemotherapy, you may have heard about Tarlatamab (Imdelltra®) as a new treatment option. But what does it actually do, and what should you know before considering this therapy? Here’s a simple breakdown to help you understand key concepts, how the drug works, and what the research means for patients like you.
If you’re a healthcare provider, access the professional version here.
How Does Tarlatamab Work?
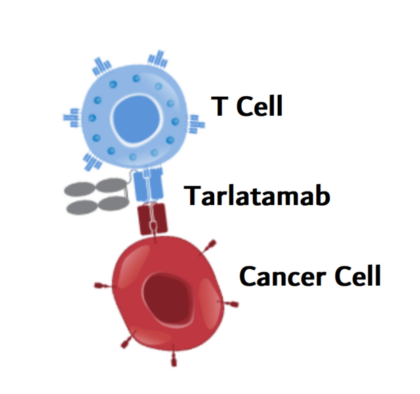
Unlike chemotherapy, which attacks both cancerous and healthy cells, Tarlatamab is a bispecific T-cell engager (BiTE®) antibody that trains the immune system to directly target cancer.
It works by binding to two key proteins:
- DLL3, a protein found almost exclusively on small cell lung cancer cells.
- CD3, a receptor on T cells (a type of immune cell responsible for fighting infections and diseases).
By bringing these two together, Tarlatamab forces the immune system to recognize and attack cancer cells, even in cases where the tumor would normally go undetected. Unlike other immunotherapies, such as PD-1 inhibitors (which remove immune system “brakes”), Tarlatamab acts more aggressively and directly, ensuring that T cells are engaged and killing cancer cells in a targeted manner.
Who Can Receive Tarlatamab?
Currently, Tarlatamab is only approved for patients with ES-SCLC who have already undergone chemotherapy. It is not yet approved for earlier stages of the disease or for other types of cancer, though ongoing trials are exploring its use in combination with other therapies, like PD-L1 inhibitors and chemotherapy.
FDA Approval and What It Means for You
Tarlatamab was approved by the U.S. Food and Drug Administration (FDA) on May 16, 2024, through a process called accelerated approval. This means that the drug showed strong early results in clinical trials, especially for patients with advanced SCLC whose cancer had worsened despite chemotherapy. Since small cell lung cancer is an aggressive disease with limited treatment options, the FDA allowed this drug to reach patients sooner while ongoing studies continue to confirm its long-term benefits.
When a drug is FDA-approved, it means that researchers have conducted clinical trials—scientific studies that test how well a treatment works, how safe it is, and how it compares to existing treatments.
Understanding Clinical Trials and What the Data Means
Tarlatamab was tested in multiple clinical trials, including DeLLphi-301, which studied its effects on patients with heavily pretreated ES-SCLC (meaning patients who had already received other treatments but whose cancer continued to grow).
The results showed promising outcomes. In this trial, 40% of patients responded to treatment, meaning their tumors shrank significantly. The median overall survival (the time when half of the patients were still alive after starting treatment) was 14.3 months, which is longer than the typical survival seen with chemotherapy in this setting. For comparison, second-line chemotherapy usually leads to a response rate of only 15% and an overall survival of around 5-9 months.
However, clinical trial results also include side effects. With Tarlatamab, one of the most common is cytokine release syndrome (CRS), a reaction where the immune system becomes overactivated, causing fever, chills, and low blood pressure. This occurred in 51% of patients at the 10 mg dose, but most cases were mild and manageable. Less commonly, some patients experienced neurological symptoms, such as confusion or difficulty speaking.
What is a Bispecific T-Cell Engager (BiTE®)?
Tarlatamab is a bispecific T-cell engager (BiTE®) antibody—but what does that mean?
- Bispecific means the drug is designed to attach to two different targets at the same time.
- T-cell engager means it helps bring the immune system’s T cells (the body’s natural defense warriors) directly to cancer cells to destroy them.
- Antibody refers to a protein that can bind to specific markers on cells, helping the immune system recognize and attack the cancer.
What Are the Most Common Side Effects of Tarlatamab?
Like all cancer treatments, Tarlatamab has potential side effects. The most common ones include:
- Cytokine Release Syndrome (CRS): A strong immune reaction that may cause fever, chills, low blood pressure, and shortness of breath.
- Neurological Effects: Some patients experience confusion, difficulty speaking, or memory issues, though these are usually temporary.
- Fatigue, nausea, and appetite loss are also frequently reported but can often be managed with supportive care.
How Is Tarlatamab Different from Traditional Immunotherapy?
Unlike conventional immunotherapies that rely on boosting the body’s general immune response, Tarlatamab actively redirects T cells to attack cancer cells in a highly targeted way. Traditional treatments, such as PD-1/PD-L1 inhibitors, work by removing immune system brakes, allowing T cells to recognize and fight tumors more effectively. However, these therapies depend on the presence of pre-activated T cells and may not work well in tumors that evade immune detection, such as small-cell lung cancer (SCLC).
Tarlatamab takes a different approach by acting as a bridge between T cells and cancer cells, ensuring direct immune engagement even in tumors that typically resist traditional immunotherapy. By binding to DLL3, a protein found almost exclusively on SCLC cells, and CD3, a receptor on T cells, Tarlatamab forces the immune system to attack, even when the tumor environment is immunosuppressive. This precision-driven mechanism makes it particularly promising for cancers like SCLC, where other immunotherapies have had limited success.
How is Tarlatamab administered?
Tarlatamab can be administered through an IV catheter that is already in use for other medications. Before infusion, the catheter should be flushed with 0.9% NaCl over 3-5 minutes to ensure patency. The medication is infused over one hour at a constant flow rate of 250 mL/hr using a programmable, lockable infusion pump equipped with an alarm for safety.
What Should You Discuss With Your Doctor?
If you are considering Tarlatamab, it’s important to have an open discussion with your oncologist. Some key questions to ask include:
- How does my cancer respond to immunotherapy?
- What are the expected benefits of Tarlatamab for my case?
- What side effects should I watch for, and how are they managed?
- Are there clinical trials I may qualify for that offer this or similar treatments?
Understanding how the drug works and interpreting clinical trial results can help you feel more confident about your treatment options. If Tarlatamab is recommended for you, it may offer a new and more effective approach to treating ES-SCLC when other treatments have failed.


