
David R. Liu: The Use of Prime Editing to Correct Several Mutations That Cause AHC
David R. Liu, Core Institute Member and Vice-Chair of the Faculty at Broad Institute of MIT and Harvard, shared on X about a recent paper by Alexander A. Sousa et al. published in Cell:
“Today in Cell we report the use of prime editing to correct several mutations that cause alternating hemiplegia of childhood (AHC), a rare and devastating neurodevelopmental disorder, in patient-derived cells and in two mouse models.

Most AHC cases are caused by mutations in ATP1A3, which encodes a sodium pump essential for neuron function. These mutations often have a dominant-negative effect; indeed, we observed that ATP1A3 gene addition in AHC mice did not improve behavioral phenotypes or survival
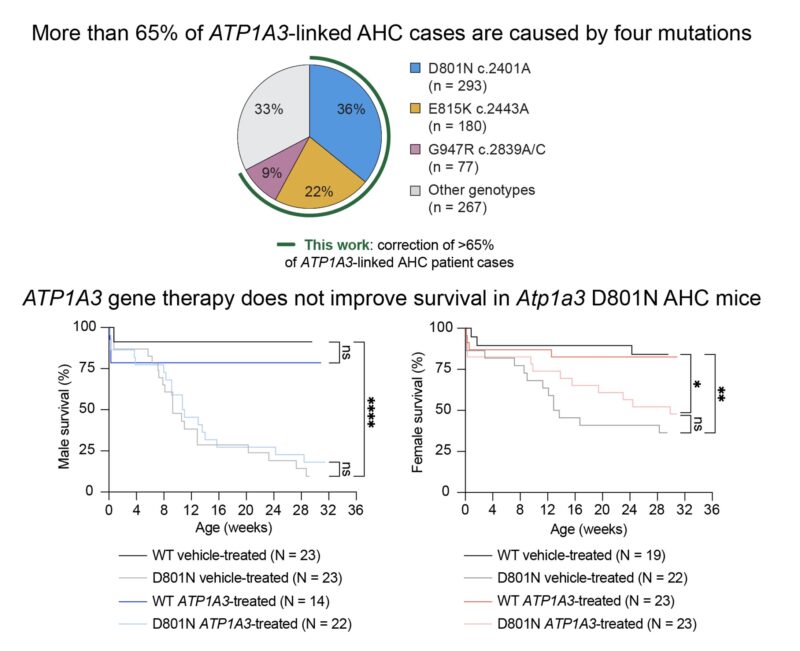
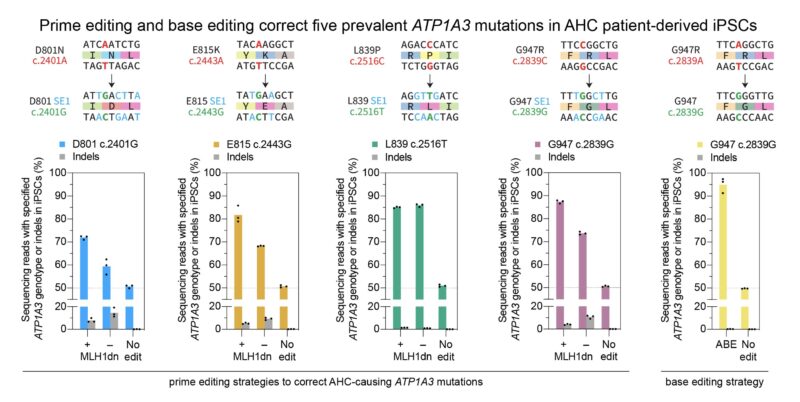
To directly address the genetic cause of AHC, we developed prime editing and base editing strategies to correct five ATP1A3 mutations, including the four most prevalent variants, with 43%–90% efficiency in AHC patient-derived iPSCs.
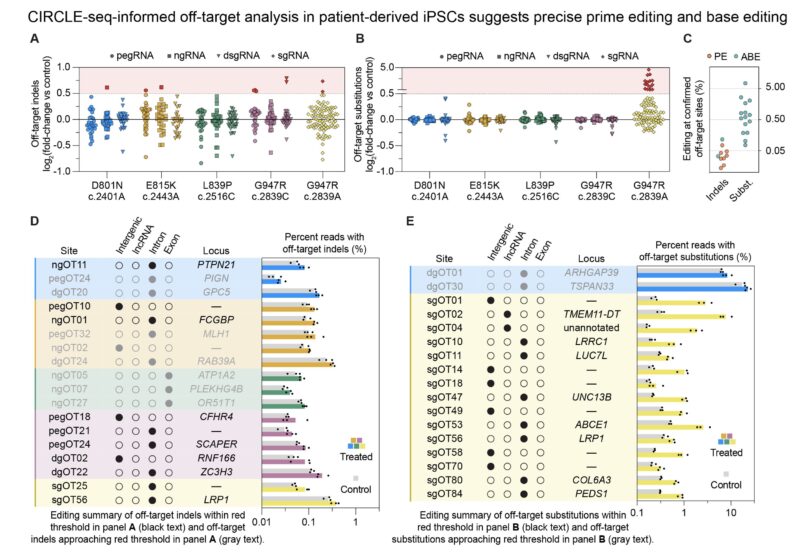
Genome-wide off-target analysis in patient-derived iPSCs detected minimal off-target editing, especially for PE and for the ABE8e-V106W or ABE7.10 variants of base editors. No off-target edits were found in exons, UTRs, splice junctions or promoters.
Next we performed in vivo brain prime editing in two AHC mouse models (Markus Terrey, Cat Lutz, et al.)
The Jackson Laboratory, PMID: 40381892). Both mouse models show key AHC features: motor/cognitive defects, paroxysmal attacks, reduced lifespan and weight, and Atp1a3 ATPase defects.
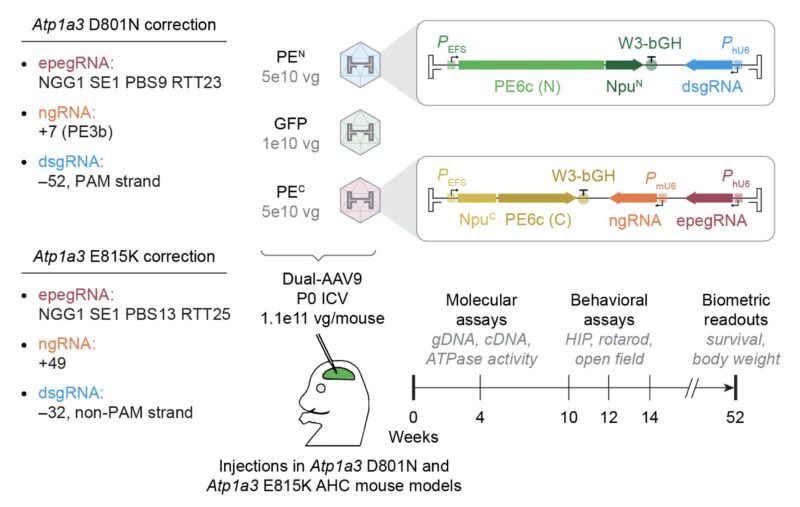
A single 1.1e11 vg/mouse P0 ICV injection of AAV9 encoding the prime editor into the brain resulted in up to 48% DNA and 73% mRNA correction in bulk cortex and rescued hippocampal Atp1a3 ATPase activity from ~50% to 77%–87% of wild-type activity in treated AHC mice.
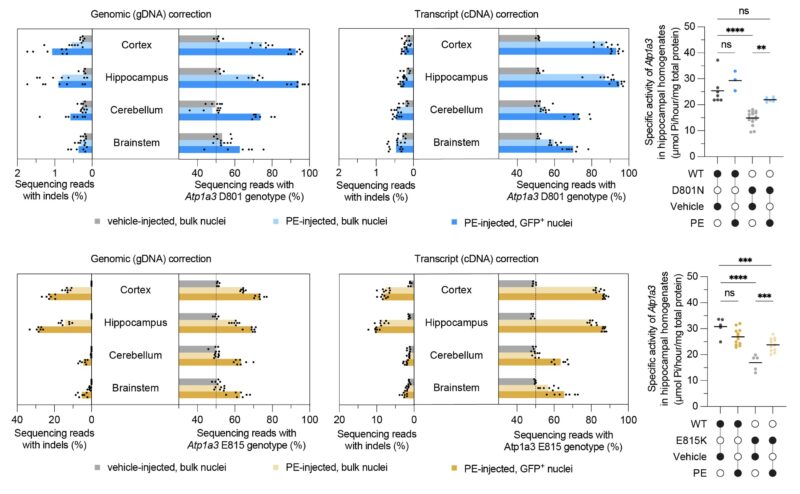
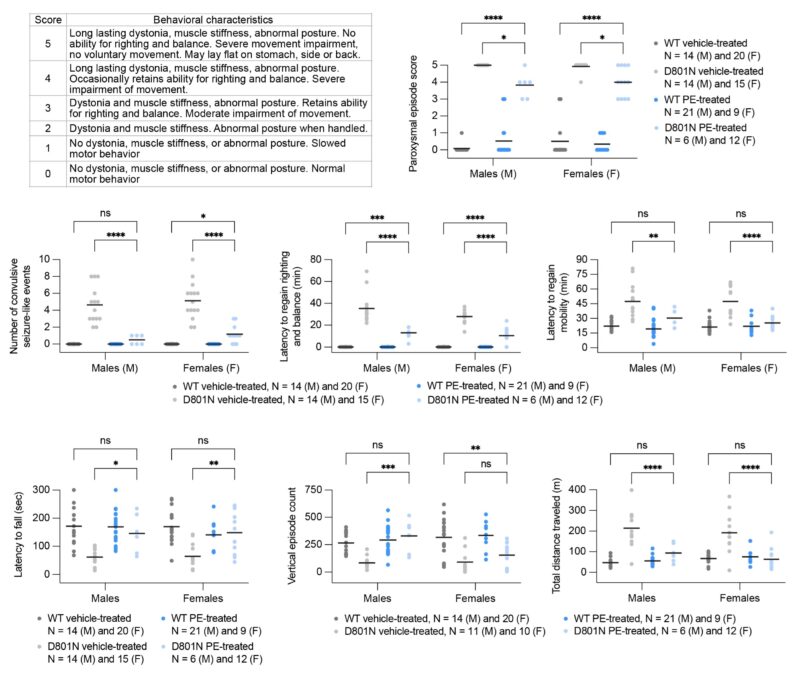
Prime editor-treated D801N (blue) and E815K (orange) AHC mice showed extensive rescue of multiple patient-relevant behavioral phenotypes, including rescue of rotarod-measured coordination to wild-type levels and near-elimination of trigger-induced seizures.
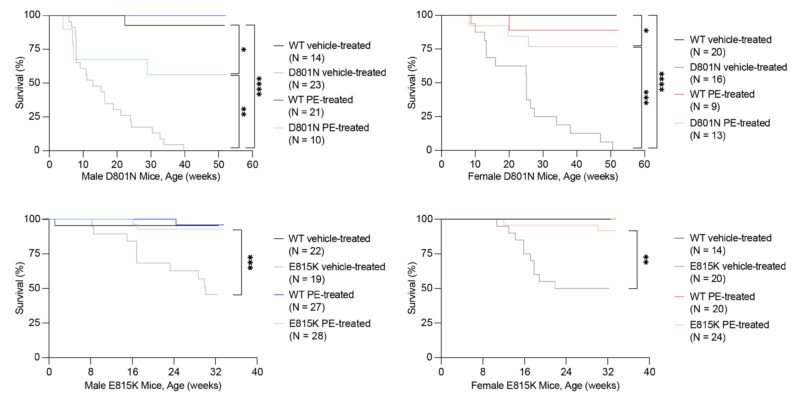
In addition to the rescue of behavioral deficits, we also observed large long-term survival improvements in prime editor-treated AHC mice compared with their vehicle-treated counterparts. Indeed, the majority of all four PE-treated mouse cohorts were still alive at the time of revised manuscript submission, 32-52 weeks after injection.
These results represent the first use of prime editing to rescue a neurological disease in animals, establish that in vivo postnatal intervention in AHC’s pathology is possible, and suggest the potential of a one-time prime editing treatment for AHC.

Research towards potential clinical use is underway. This work was co-led by Alexander Sousa, Holt Sakai and Markus Terrey and co-directed with Cat Lutz, in a wonderful collaboration of Broad Institute, The Jackson Laboratory, RARE Hope, Northwestern, Mass General BrighamUMass Chan Medical School.”
Authors: Alexander A. Sousa, Markus Terrey, Holt A. Sakai, Christine Q. Simmons, Elena Arystarkhova, Natalia S. Morsci, Laura C. Anderson, Jun Xie, Fabian Suri-Payer, Linda C. Laux, Emmanuel Roze, Sylvie Forlani, Guangping Gao, Simon Frost, Nina Frost, Kathleen J. Sweadner, Alfred L. George, Jr., Cathleen M. Lutz, David R. Liu
More posts featuring Childhood Cancer.
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
