May 2024 Marks The 20th Anniversary of the Publication of Papers on The Role of the EGFR Mutation in Lung Cancer.
The discovery of the epidermal growth factor receptor (EGFR) and its role in cancer began with research in the 1970s and 1980s. In 1984, Stanley Cohen, a Vanderbilt University scientist, discovered that EGFR was a transmembrane protein that could bind to growth factors, triggering cellular growth and proliferation. This work earned Cohen the Nobel Prize in Physiology or Medicine in 1986, which he shared with Rita Levi-Montalcini for their discoveries in the field of growth factors.
However, EGFR’s pivotal role in cancer was fully realized later, especially in lung cancer research. In May 2004, two landmark studies dramatically changed the understanding of EGFR in non-small-cell lung cancer (NSCLC). A publication by Lynch et al. in The New England Journal of Medicine identified specific mutations in the EGFR gene, primarily in exons 19 and 21, that were associated with a clinical response to the EGFR tyrosine kinase inhibitor (TKI) gefitinib. This discovery led to the development of targeted therapies for EGFR-mutant NSCLC and significantly altered treatment strategies.
The findings were reinforced by other studies, such as those by Paez et al. and Pao et al., showing that these EGFR mutations were particularly prevalent in non-smokers, with higher response rates to EGFR inhibitors in Asian populations. This marked a crucial turning point in cancer treatment and was a key factor in the decline of cancer mortality in the U.S. Since then, EGFR mutations have become a critical biomarker for treatment decisions in lung cancer, with several EGFR inhibitors, such as gefitinib, erlotinib, afatinib, and osimertinib, being developed and approved for clinical use.
About the epidermal growth factor receptor (EGFR)
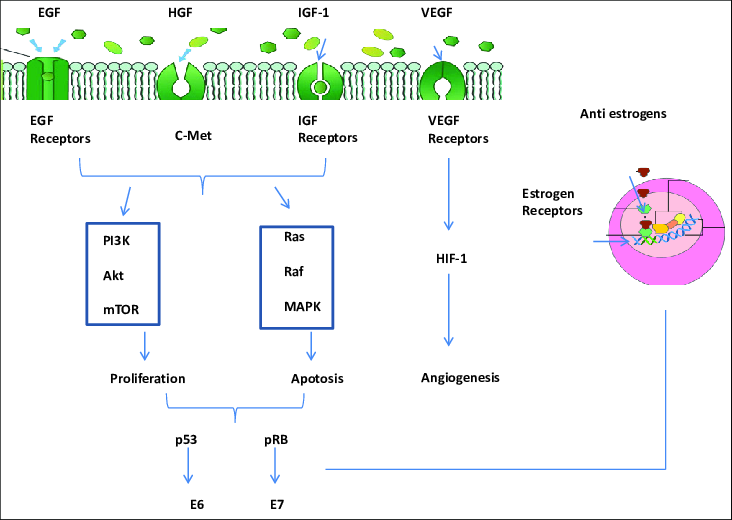
The epidermal growth factor receptor (EGFR) is a transmembrane protein that plays a critical role in regulating cell growth, survival, and differentiation. It belongs to the ErbB family of receptor tyrosine kinases, which also includes HER2/neu (ErbB-2), HER3 (ErbB-3), and HER4 (ErbB-4). EGFR is activated when specific ligands, such as epidermal growth factor (EGF) and transforming growth factor-alpha (TGF-α), bind to its extracellular domain. This binding causes a conformational change in the receptor, leading to its activation and the initiation of downstream signaling pathways that regulate essential cellular processes, including cell proliferation, survival, and migration. Dysregulated EGFR signaling is implicated in various cancers, making it an important target for cancer therapies.
In cancer, EGFR overexpression or mutations can lead to continuous activation of the receptor, promoting uncontrolled cell growth and tumor progression. This aberrant signaling is a hallmark of several cancer types, including non-small-cell lung cancer (NSCLC), colorectal cancer, and breast cancer. Targeted therapies, such as EGFR tyrosine kinase inhibitors (TKIs) and monoclonal antibodies, have been developed to block EGFR activation and disrupt the downstream signaling pathways. These therapies aim to prevent tumor growth and improve patient outcomes. However, resistance to EGFR-targeted treatments can develop due to mutations in the receptor or activation of alternative signaling pathways, presenting a significant challenge in the treatment of EGFR-driven cancers.
Upon activation, EGFR undergoes dimerization, either forming homodimers or heterodimers with other ErbB family members, such as HER2. This dimerization activates EGFR’s intrinsic tyrosine kinase activity, leading to the phosphorylation of tyrosine residues in the receptor’s cytoplasmic tail. These phosphorylated residues act as docking sites for downstream signaling proteins, triggering the activation of key pathways like MAPK, Akt, and JNK. These pathways control various cellular processes, including DNA synthesis and cell proliferation. Understanding the intricate signaling networks involving EGFR and related receptors has led to the development of targeted therapies, though overcoming resistance mechanisms remains an ongoing challenge in treating EGFR-driven cancers.
The Paper published on Nature Portfolio titled “The changing treatment landscape of EGFR-mutant non-small-cell lung cancer” is about Third-generation EGFR TKIs that have become the standard first-line treatment for advanced-stage EGFR-mutant NSCLC, with their potent central nervous system penetrance offering particular benefits for patients with brain metastases. These advancements have significantly improved clinical outcomes and provided a more targeted treatment approach for this specific subgroup of NSCLC patients.
Authors: Fei Zhou, Haoyue Guo, Yang Xia, Xiuning Le, Daniel S. W. Tan, Suresh S. Ramalingam, Caicun Zhou

Despite the progress made with EGFR TKIs, acquired resistance remains a major challenge in the management of EGFR-mutant NSCLC. To address this, researchers are investigating various strategies to delay or overcome resistance. These include rational combinations of third-generation EGFR TKIs with other therapies such as anti-angiogenic drugs, chemotherapy, and the EGFR-MET bispecific antibody amivantamab. Additionally, local tumor ablation techniques are being explored as complementary strategies to enhance the clinical benefit and extend the duration of response in patients.
Moreover, there is increasing interest in applying EGFR TKIs in earlier-stage or locally advanced EGFR-mutant NSCLC, with the ambitious goal of achieving a potential cure. While the current treatment paradigm primarily focuses on advanced disease, ongoing studies are evaluating the use of EGFR TKIs in the adjuvant or neoadjuvant settings to improve long-term survival outcomes and possibly eradicate early-stage disease. These strategies could represent a significant shift toward more curative treatments for patients with EGFR-mutant NSCLC.
The review also highlights the emerging treatments aimed at overcoming EGFR TKI resistance, which remains a critical obstacle. New-generation TKIs, antibody-drug conjugates, immunotherapies, and targeted protein degraders show promising early results in patients who have progressed on or after first-line EGFR TKI therapy. These innovative approaches provide hope for improving survival outcomes in EGFR-mutant NSCLC patients who have developed resistance. Ongoing research and clinical trials will be pivotal in determining the future of EGFR TKI treatment and its potential to improve patient outcomes and survival rates.
Introduction of the Abstract
Lung cancer remains a leading global health challenge, primarily due to its molecular complexity, which presents significant barriers to effective treatment. Among the various subtypes, EGFR-mutant non-small-cell lung cancer (NSCLC) has emerged as a major focus in precision oncology research. The discovery of mutations in the EGFR gene (which encodes a receptor from the ERBB family) has transformed the treatment landscape for this disease. These mutations are present in about 20%-30% of NSCLC cases in white patients and in approximately 50% of Asian patients. This has paved the way for the development of molecularly targeted therapies, which have significantly improved patient outcomes and quality of life.
Over the last decade, significant strides have been made in understanding the molecular mechanisms driving EGFR-mutant NSCLC, leading to the development of innovative therapeutic strategies. EGFR tyrosine kinase inhibitors (TKIs) have proven highly effective in inhibiting EGFR signaling and triggering antitumor responses. Additionally, advances in next-generation sequencing (NGS) have facilitated the identification of new molecular markers, providing valuable prognostic and predictive biomarkers that enhance personalized medicine approaches, ultimately improving patient outcomes. However, despite these advances, the challenge of acquired resistance to EGFR TKIs remains a significant concern, necessitating continued exploration of new therapeutic strategies.
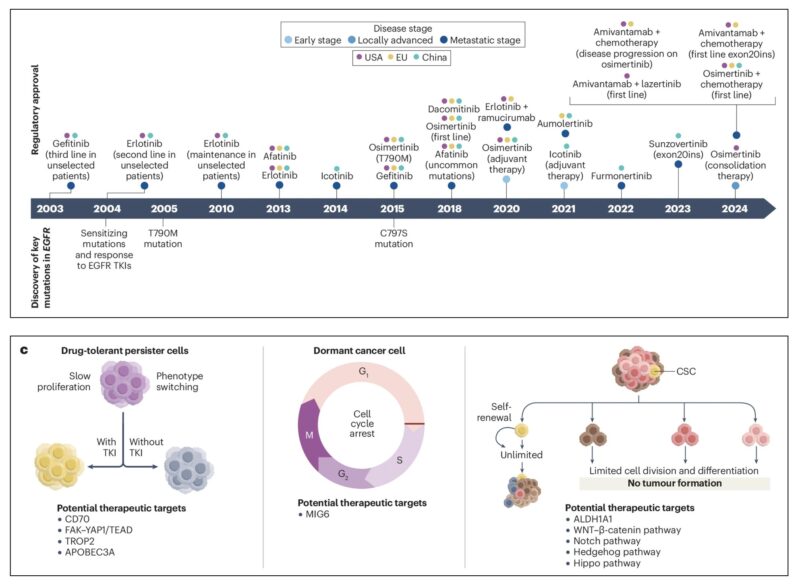
Recent research has focused on unraveling the mechanisms behind resistance to EGFR TKIs, including secondary mutations in EGFR, activation of alternative signaling pathways, and histological transformations, all of which contribute to disease progression during EGFR TKI treatment. Encouragingly, the development of biomarker-guided combination therapies has shown promise in overcoming resistance and improving treatment responses in patients with EGFR-mutant NSCLC. These combination strategies are helping to address the pressing issue of resistance and may offer more durable clinical benefits for patients.
As ongoing research continues to decode the complexities of EGFR-mutant NSCLC, the quest for more effective treatments with lasting efficacy continues. The routine clinical use of EGFR TKIs has not only expanded the range of treatment options in metastatic settings but has also raised hopes for achieving curative outcomes in patients with early-stage or locally advanced EGFR-mutant NSCLC. This review synthesizes the latest findings in this rapidly evolving field, providing insights into the role of precision medicine in the treatment of EGFR-mutant NSCLC and outlining promising future directions for research and clinical practice in this area.
About Osimertinib
Osimertinib, marketed under the brand name Tagrisso, is a third-generation epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) used primarily for the treatment of non-small-cell lung cancer (NSCLC) in patients with specific EGFR mutations. It was developed to target EGFR mutations that are common in lung cancer, particularly the T790M mutation, which can develop as a resistance mechanism following treatment with first-line EGFR TKIs such as gefitinib, erlotinib, or afatinib. Osimertinib is approved for use in patients with locally advanced or metastatic NSCLC who test positive for these mutations, offering a more effective option for those who have developed resistance to other EGFR-targeted therapies.
The drug works by irreversibly binding to the EGFR tyrosine kinase receptor, inhibiting its signaling pathways, which are critical for the growth and survival of cancer cells. Osimertinib’s ability to penetrate the blood-brain barrier makes it particularly effective for treating brain metastases, a common complication in patients with advanced NSCLC. This has positioned osimertinib as a valuable treatment option for patients with EGFR-mutant NSCLC who also experience central nervous system involvement.
Approved for medical use in the United States in November 2015 and in the European Union in February 2016, osimertinib has become a cornerstone in the treatment of EGFR-mutant NSCLC. The drug was initially approved based on its ability to address the T790M mutation, a mutation that develops in about half of patients with resistance to earlier EGFR TKIs. In February 2024, the FDA extended the approval of osimertinib for use in combination with platinum-based chemotherapy for patients with locally advanced or metastatic NSCLC harboring EGFR exon 19 deletions or exon 21 L858R mutations.
Despite its effectiveness, resistance to osimertinib typically develops within about ten months of treatment. A common mechanism of resistance is the emergence of a C797S mutation in the EGFR gene, which can disrupt osimertinib’s binding to the EGFR receptor. To address this challenge, researchers are exploring non-ATP competitive and allosteric inhibitors to overcome this resistance. In September 2024, the FDA further expanded osimertinib’s indication to include patients with locally advanced, unresectable (stage III) NSCLC who have not progressed after platinum-based chemoradiation therapy, further solidifying its role in the management of advanced NSCLC.