Photo taken from Amin Nassar/X
Amin Nassar, Hematology/oncology fellow at Yale University, shared a post on X/Twitter:
“Pumped to share that our article ‘Consolidation Osimertinib versus Durvalumab versus Observation following Concurrent Chemoradiation in Unresectable EGFR-Mutant Non-Small-Cell Lung Cancer: A Multicenter Retrospective Cohort Study’ is now out in JTO!
So thankful to be part of a SPLENDID team So Yeon Kim, Elio Adib, De’Andre Nash and Sarah Goldberg.
The PACIFIC Trial transformed the treatment paradigm for patients with stage III unresectable NSCLC by demonstrating improved progression free survival (PFS) and overall survival (OS) with consolidation durvalumab compared to placebo. This is further shown by real-world data
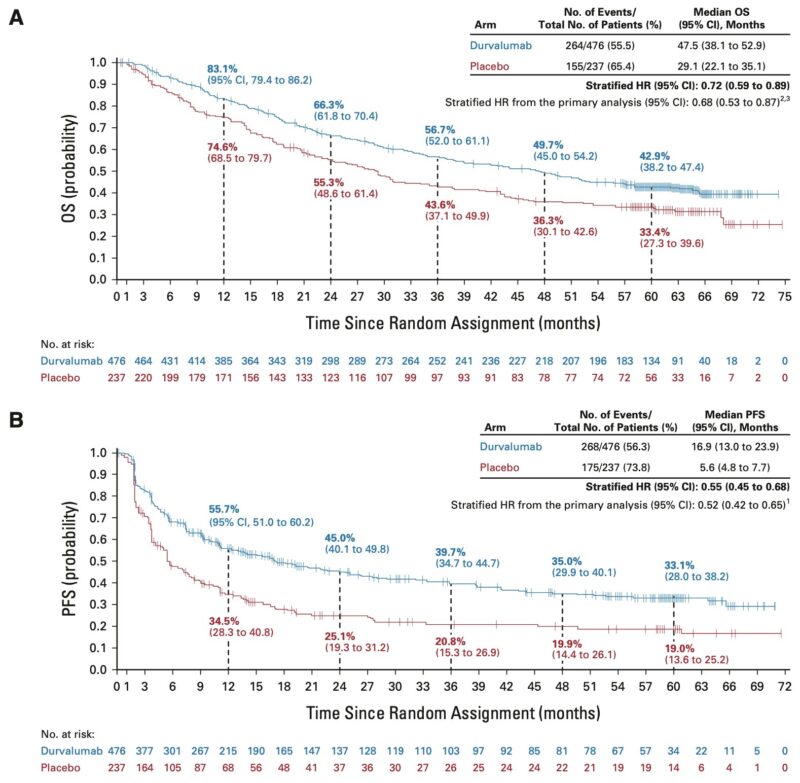
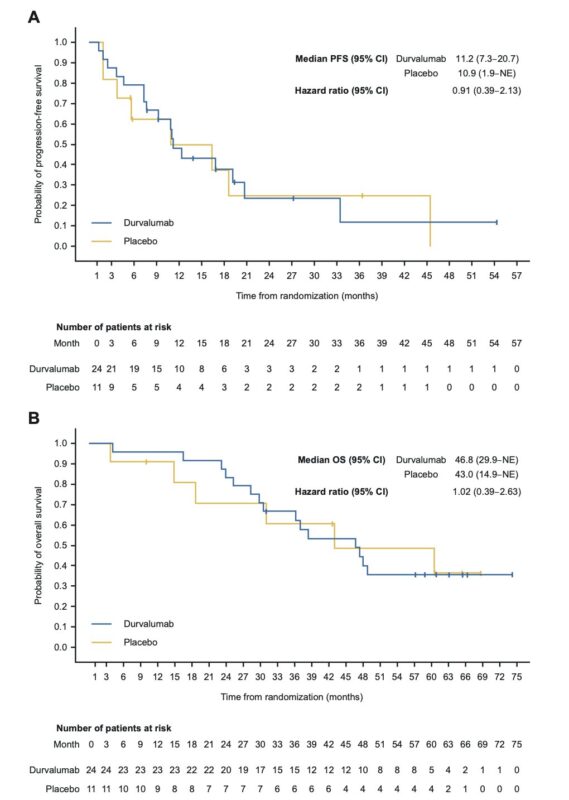
Immunotherapy has demonstrated less clinical efficacy in EGFR-mutant NSCLC (EGFRm) in the Stage III and metastatic settings. Data in the Stage III setting below by Jarushka Naidoo et al
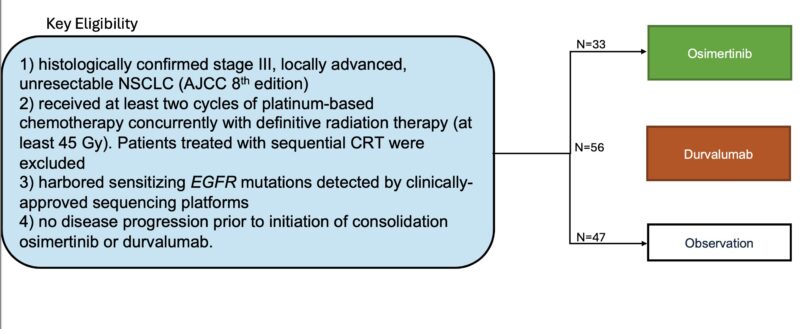
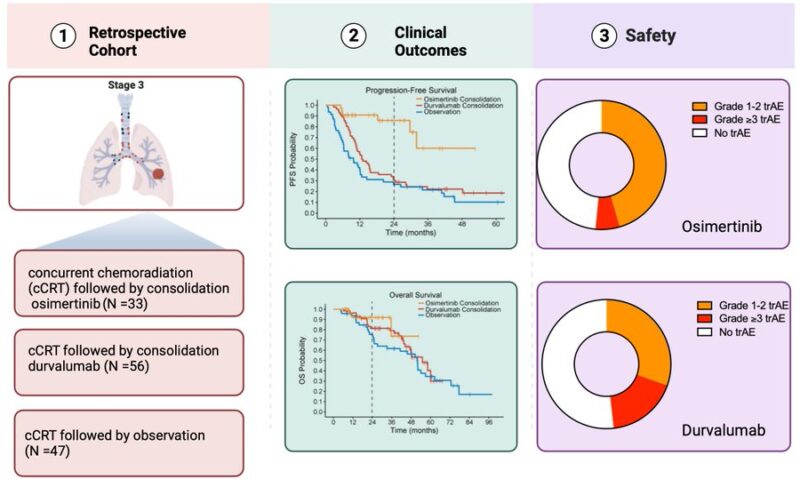
Osimertinib has demonstrated superior OS and DFS compared to placebo in resectable stage III NSCLC (ADAURA). How about in unresectable stage III NSCLC? We set out to answer this in this multi-center retrospective cohort composed of 24 institutions
Inclusion Criteria below:
Now to the results: no differences in baseline characteristics across the 3 treatment cohorts. The median time on osimertinib was not reached (95% CI NR-NR), whereas the median time on durvalumab was 5.5 (IQR:2.4-10.8) months. Three out of 33 patients treated with consolidation osimertinib continued on treatment beyond the 3-year mark. Six and 10 patients crossed the 2-year and 1-year marks, respectively.
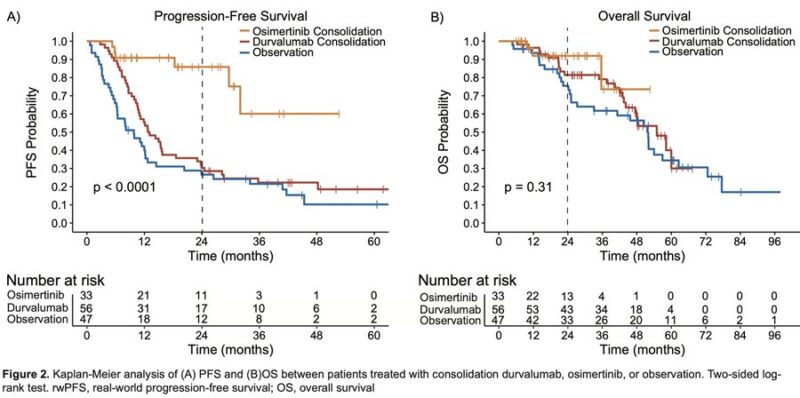
The median rwPFS was not reached (95% CI, NR-NR) in the osimertinib cohort, 12.7 months (95% CI, 10.5 to 15.5) in the durvalumab cohort, and 9.7 months in the observation cohort (95% CI, 6.1 to 12
2y rwPFS rate 86% with osimertinib, 30% w/ durvalumab, 27% w/ observation
HR(osimertinib-durvalumab) = 0.20; HR(osimertinib-observation)= 0.14; HR (durvalumab-observation) = 0.67
Recognizing limited follow-up, there was no significant difference in median OS across the 3 cohorts. Median OS was not reached in the osimertinib cohort (95% CI NR-NR), 54 months in the durvalumab cohort (95% CI 46-NR), and 51 months in the observation cohort (95% CI 32-71 months).
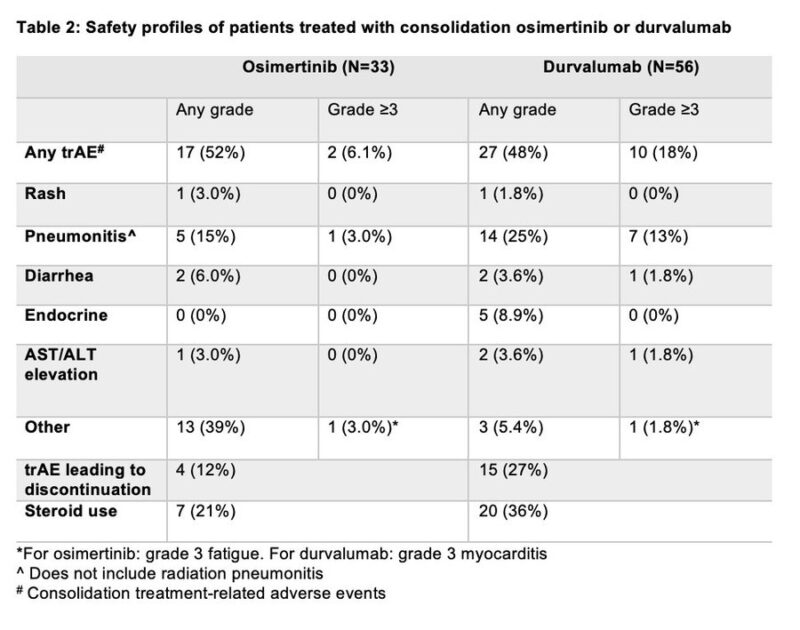
Treatment-related adverse events are shown below
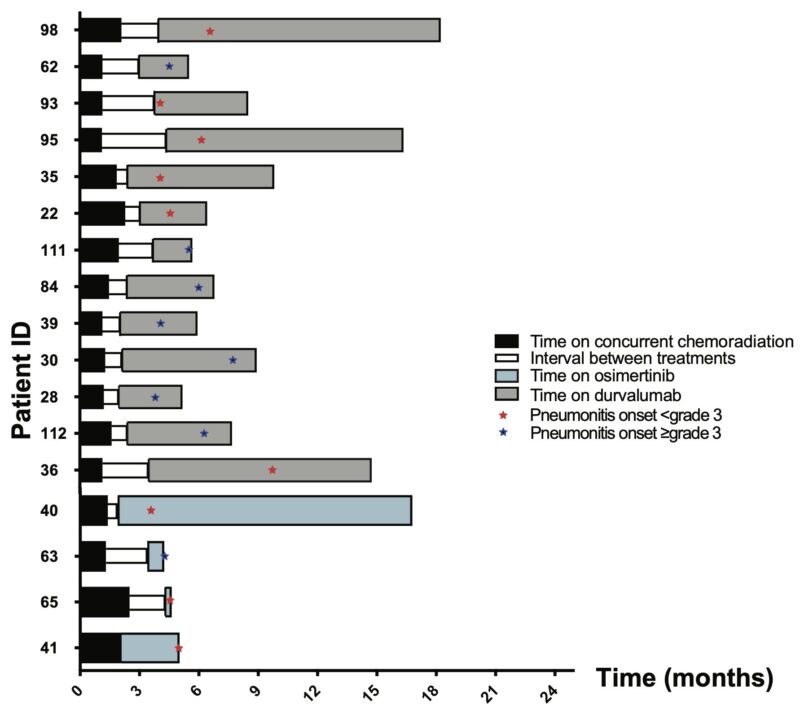
The incidence of early-onset pneumonitis (up to 90 days from the start of consolidation) was 4/33 (12%) and 9/56 (16%) among patients treated with osimertinib and durvalumab, respectively. Late-onset pneumonitis (90-180 days from the start of consolidation treatment) occurred in 1/33 (3%) patients and 3/56 (5.4%) of patients treated with osimertinib and durvalumab, respectively.
Conclusion in graphical abstract below
Future directions: Evaluating the optimal timeline, duration of consolidation treatment, and washout period from CRT initiation to consolidation osimertinib to optimize safety and efficacy. Let’s wait for LAURA (note comparator arm there is placebo and experimental arm is indefinite Osi if tolerated and no progression)!!”