
Katsuaki Maehara: Patritumab Deruxtecan (HER3-DXd) clinical trial transition
Katsuaki Maehara, Director of Medical Education Colleagues, recently shared a post by EGFR Resisters on X/Twitter, adding:
”Patritumab Deruxtecan (HER3-DXd). Clinical Trial Transition. Introduce.

Clinical Trial Transition Part-1-1. Phase 1 study. Pasi Jänne – ASCO 2021.

Clinical Trial Transition Part-1-2. Phase 1 study – Efficacy and Toxicity.
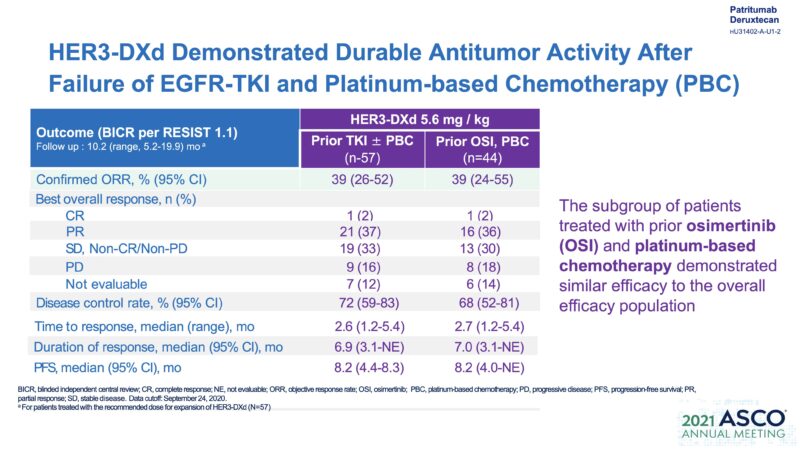
Clinical Trial Transition Part-2-1. Phase 1 study – Without EGFR mutation.

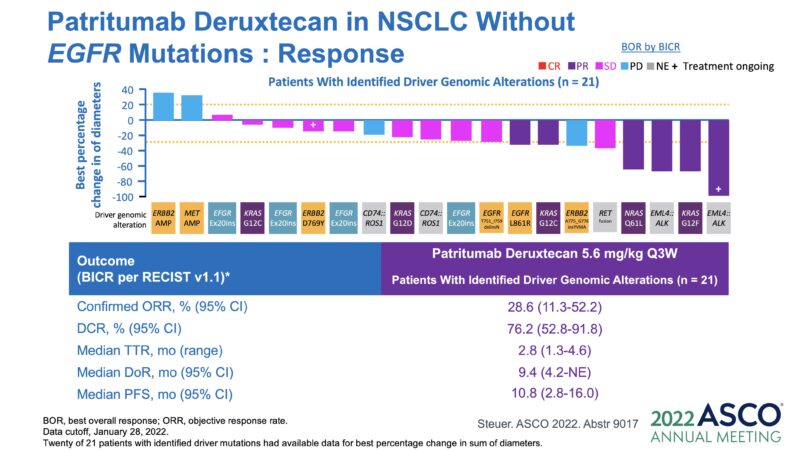
Clinical Trial Transition Part-2-2. Phase 1 study – Without EGFR mutation. Efficacy and Toxicity.
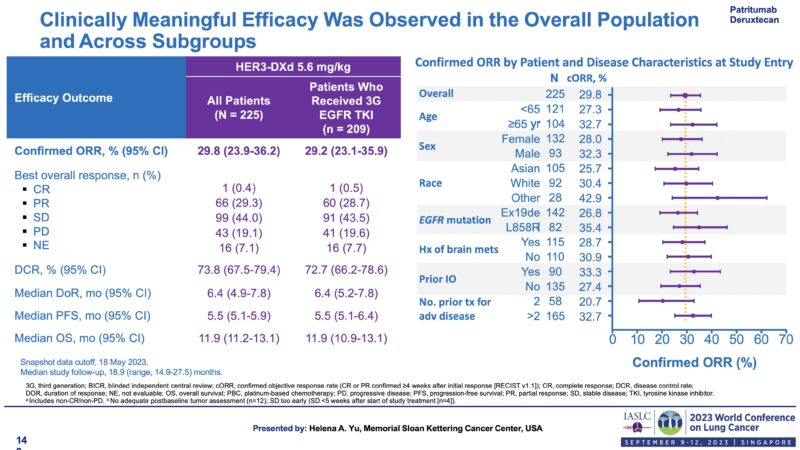
Clinical Trial Transition Part-3-1. Phase 2 study Helena Yu– WCLC 2023.

Clinical Trial Transition Part-3-2. Phase 2 study – Efficacy (inc CNS).
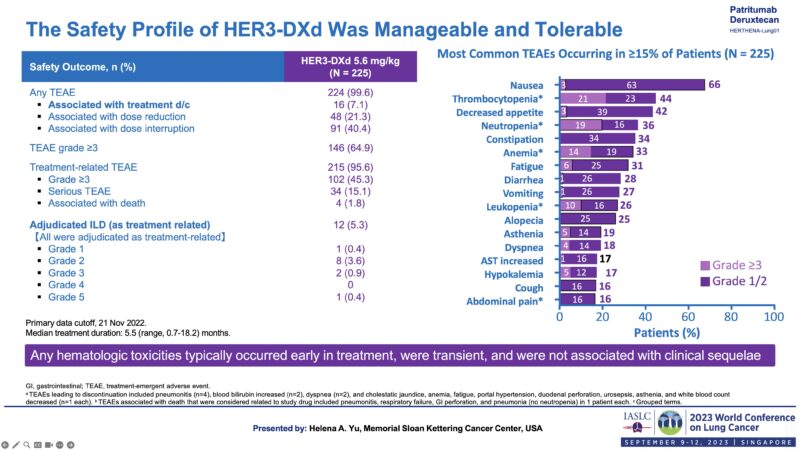
Clinical Trial Transition Part-3-3. Phase 2 study -Toxicity.
Clinical Trial Transition Part-3-3. Phase 2 study from Journal of Clinical Oncology, Helena Yu. Efficacy.

Clinical Trial Transition Part-3-4. Phase 2 study from Journal of Clinical Oncology, Helena Yu. Toxicity and EGFR TKI resistance associated genomic alterations (Efficiency population). Read further.
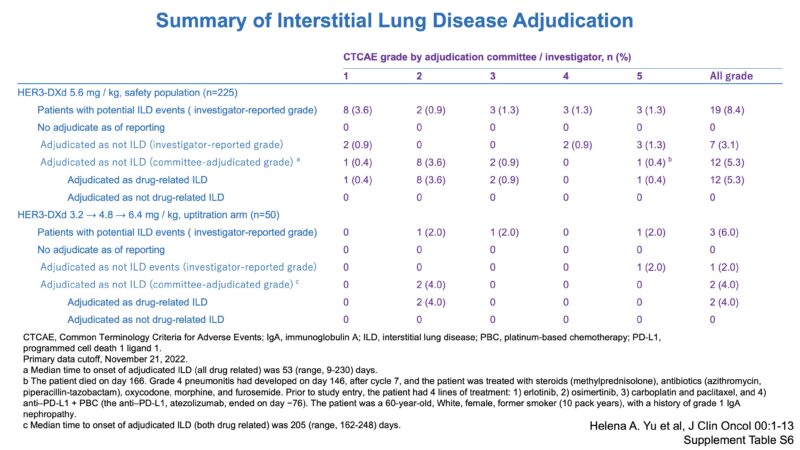
Clinical Trial Transition Part-4-1. Intracranial Efficacy Melissa Johnson. ESMO23.

Clinical Trial Transition Part-4-2. Intracranial Efficacy.
Combination with Osimertinb Part-5. Paper suggests that osimertinib in combination with HER3-DXd may be an effective therapeutic approach. Heidi Haikala, Pasi Jänne et al Cancer Research, AACR. Read further.
Ongoing Clinical Trials. HERTHENA-Lung02. HER3-DXd in Combination With Osimertinib. Read further. Read further.”
Quoting EGFR Resisters’s post:
”Patritumab Deruxtecan Granted Priority Review in the U.S. for Certain Patients with Previously Treated Locally Advanced or Metastatic EGFR-Mutated Non-Small Cell Lung Cancer.”
Source: Katsuaki Maehara/X and EGFR Resisters/X
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
