Michael T. McCarthy posted on X:
“A case study of Anti-Cancer Drug reimbursement in Ireland
Background:
Dostarlimab is an immunotherapy that binds to PD-1 on T cells, and prevents PDL1 expressed on tumour cells from ‘switching off’ T cells. In other words, in theory, when a T lymphocyte (and maybe other classes of immune cells) meets a cancer cell expressing PDL1, the addition of dostarlimab – on paper – tips the balance in favour of the immune cell reacting against/eliminating the cancer cell.
Several clinical trials have demonstrated that dostarlimab improves outcomes for people with a range of incurable cancers. Take the example here of advanced incurable endometrial cancer that is deficient for Mismatch Repair Proteins (dMMR) or the equivalent functional characteristic of having high microsatellite instability, having progressed on standard of care first line chemotherapy with carboplatin and paclitaxel. Interim results of a phase 1 clinical trial have been published (link here). The overall response rate was 44%, and ~40% had no evidence of disease progression at 36 months into treatment.
Context:
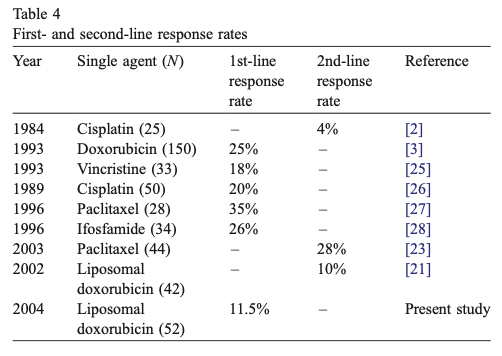
Compared to the alternative treatment options currently available (second line palliative chemotherapy), the outcomes from the dostarlimab trial represent a clear step-change improvement on what has been available to date. For example, in the case of liposomal doxorubicin, here is a summary table from a phase 2 clinical trial. This was published in 2005. The study showed that in using liposomal doxorubicin in the first line, the response rate was 11.5% (compared to 44% second line with dostarlimab).
The median overall survival in this study was 10.9 months (compared to a 3 year progression free survival rate of ~40%). These statistics are not directly comparable, there are many differences between these two studies, but if dostarlimab and liposomal doxorubicin were compared head-to-head in a RCT for advanced dMMR endometrial cancers, the outcomes would most likely be worlds apart as far as I can see.
On the basis of the dostarlimab phase 1 clinical trial, the EU Medicines Agency has approved dostarlimab as a therapy option for advanced dMMR endometrial cancer patients.
When the EMA issues approval for a drug it considers the following:

There has been an independent academic (as far as I am aware the pharmaceutical company did not pay for this overtly, it is not declared in the conflicts of interest at least) cost-effectiveness analysis published for the use of dostarlimab compared to the standard of care alternative option for second line therapy in advanced endometrial cancer, liposomal doxorubicin. Here it is.
The NCPE has also assessed dostarlimab for reimbursement for Irish cancer patients with advanced dMMR endometrial cancer. The NCPE concluded that the cost-effectiveness of dostarlimab was insufficient to approve reimbursement. I let price negotiations to others, but I do note that the ‘standard of care’ described in the tables below from the NCPE is more than 20 years old, and has a response rate of 11.5% in first line, compared to the 44% response rate of dostarlimab in this population.
To be very clear, this drug does work extraordinarily very well for many patients with advanced dMMR endometrial cancer. Because it is not ‘cost-effective’ relative to the best treatment the 20th century had to offer, it has not been approved for reimbursement.
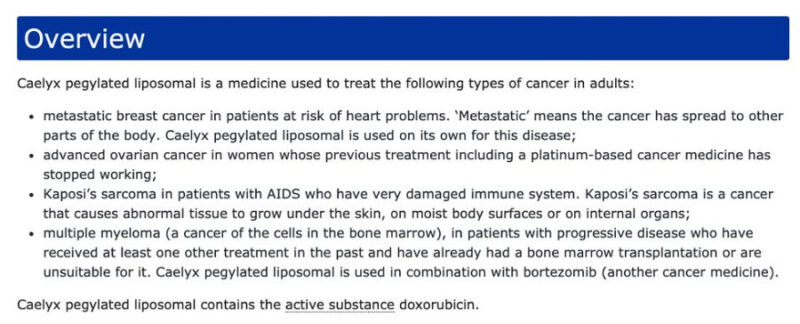
Ironically, liposomal doxorubicin, the chemotherapy that will be most commonly used in place of dostarlimab for public cancer patients with advanced dMMR endometrial cancer is not approved by the EMA for that indication. Here is the list of indications approved by the EMA for liposomal doxorubicin:
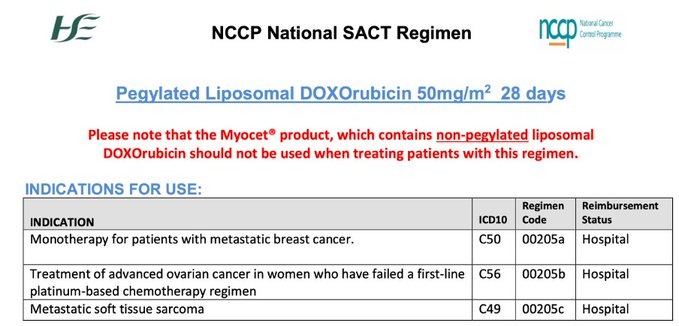
Liposomal doxorubicin is also not approved for use in advanced endometrial cancer by the HSE. Here are the currently HSE-approved indications for this drug:
Summary: Dostarlimab is very effective in treating advanced dMMR endometrial cancer. The public healthcare system in Ireland cannot afford this drug for public cancer patients (it has been fully assessed by the HSE reimbursement process and declined). Private cancer patients can access this treatment. In public hospitals we instead will continue to use less effective therapies, that are not EMA approved.”
Michael McCarthy is a Consultant Medical Oncologist at University Hospital Galway, with a focus on immunology and its applications in oncology. He previously served as a Locum Consultant Medical Oncologist at St. James’s Hospital Dublin and Letterkenny University Hospital.


