On August 2, 2024, the FDA made history by granting accelerated approval to afamitresgene autoleucel (TECELRA), the first-ever engineered cell therapy for a solid tumor.
This groundbreaking treatment, developed by Adaptimmune, LLC, offers new hope for adults battling unresectable or metastatic synovial sarcoma who have already undergone chemotherapy.
Afami-cel, as it’s commonly known, represents a paradigm shift in cancer care, utilizing the patient’s own genetically re-engineered immune cells to target and destroy cancer cells.
This precision medicine approach, tailored for specific HLA types and tumors expressing the MAGE-A4 antigen, marks the first significant advancement in synovial sarcoma treatment in over a decade.

Sandra D’Angelo, who is the principal investigator for the SPEARHEAD-1 trial, which supported the FDA’s approval, shared on her Linkedin (@sandrapdangelo)
“Afami-cel, TCR targeting MAGEA-4 approved for synovial sarcoma. A remarkable advancement for our patients, laying the foundation for future efforts in solid tumor.”
What is Cell Therapy and How Does Afamitresgene Autoleucel Work?
Understanding Cell Therapy
T cell therapy is a groundbreaking immunotherapy approach that harnesses the power of a patient’s own immune system to fight cancer. The process begins by extracting T cells, a type of white blood cell crucial for immune response, from the patient’s blood.
These cells are then genetically modified in a laboratory to enhance their ability to recognize and attack specific cancer cells. The modification often involves equipping the T cells with chimeric antigen receptors (CARs) or T cell receptors (TCRs) that can identify cancer-specific antigens. Once modified, these T cells are multiplied in the lab to create a large army of cancer-fighting cells.
Before reinfusion, the patient undergoes lymphodepletion chemotherapy to create space for the engineered T cells. When reintroduced into the patient’s bloodstream, these specially engineered T cells seek out and attack cancer cells expressing the target antigen, potentially leading to tumor regression.
What is Afamitresgene Autoleucel?
Afamitresgene autoleucel, marketed under the brand name TECELRA, is an advanced cell therapy developed by Adaptimmune, LLC, for the treatment of advanced synovial sarcoma. It represents a significant advancement in the field of solid tumor immunotherapy.

How does it Afami-Cel work?
Afamitresgene autoleucel is a type of engineered T cell receptor (TCR) therapy. Here’s how it works:
- T cell modification: The patient’s own T cells are extracted and genetically engineered to express a specific T cell receptor.
- MAGE-A4 targeting: The engineered receptor is designed to recognize the MAGE-A4 antigen, which is commonly expressed in synovial sarcoma cells.
- Enhanced tumor recognition: When reinfused into the patient, these modified T cells can more effectively identify and attack MAGE-A4-expressing cancer cells.
- Immune system activation: The therapy stimulates a targeted immune response against the tumor.
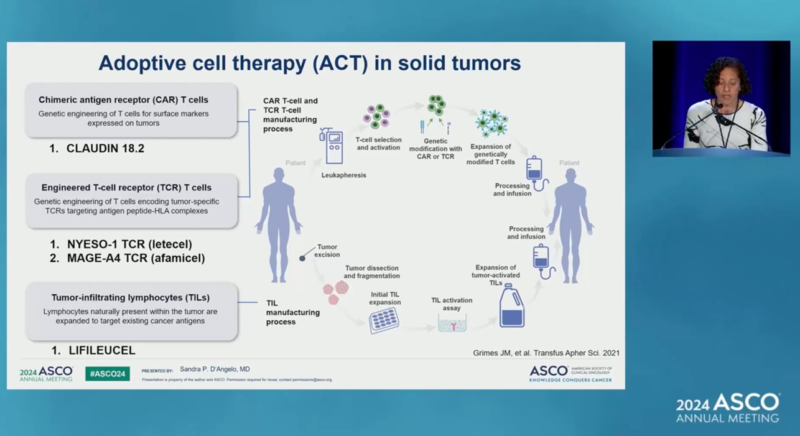
Sandra D’Angelo, Immunotherapy in Sarcoma: Current Data and Promising Strategies, Presented at ASCO 2024
Administration and Dosage
Afamitresgene autoleucel is administered as a single intravenous infusion. The recommended dose ranges from 2.68 x 10^9 to 10 x 10^9 MAGE-A4 TCR positive T cells. The specific dosage is determined based on the patient’s individual characteristics and disease state.
Key points about administration:
- One-time treatment: Unlike many cancer therapies, afamitresgene autoleucel is designed to be a single-dose treatment.
- Preconditioning: Patients typically receive lymphodepleting chemotherapy before the infusion to enhance the therapy’s effectiveness.
- Specialized centers: Due to the complex nature of cell therapy, treatment is usually administered at specialized healthcare facilities.
Eligibility and Patient Selection
Not all synovial sarcoma patients are eligible for this therapy. Key criteria include:
- HLA type: Patients must be HLA-A02:01P, -A02:02P, -A02:03P, or -A02:06P positive.
- MAGE-A4 expression: The tumor must express the MAGE-A4 antigen.
- Prior treatment: Patients should have received prior chemotherapy.
- Disease stage: Intended for unresectable or metastatic synovial sarcoma.
Side Effects and Safety Considerations
As with many powerful immunotherapies, afamitresgene autoleucel can cause significant side effects. The most notable include:
- Cytokine Release Syndrome (CRS): A potentially severe inflammatory response that requires close monitoring and management.
- Neurological toxicities: Including immune effector cell-associated neurotoxicity syndrome (ICANS).
- Hematological effects: Such as decreased blood cell counts.
- Infections: Due to the immunosuppressive nature of the preconditioning regimen.
Understanding Synovial Sarcoma: A Rare and Challenging Soft Tissue Cancer
Synovial sarcoma is a rare and aggressive type of soft tissue sarcoma thatprimarily affects the extremities, often near the joints of the arms and legs. Despite its name, synovial sarcoma does not originate from synovial tissuebut rather from mesenchymal cells that can differentiate into various tissue types.
This malignancy is characterized by a specific chromosomal translocation, making it one of the well-defined translocation-associated sarcomas. It is a malignant neoplasm that accounts for approximately 5-10% of all soft tissue sarcomas.
It predominantly affects younger adults and children, with a peak incidence in the fourth decade of life. This cancer is characterized by its unique genetic signature, with over 95% of cases harboring a specific chromosomal translocation between chromosomes X and 18, resulting in the formation of SS18::SSX fusion oncogenes.
Key Features of Synovial Sarcoma:
- Incidence: Approximately 3 cases per million people per year
- Age Range: Can occur at any age, but most common in teens and young adults (15-35 years)
- Common Sites: Soft tissues around hip, knee, ankle, or shoulder joints
- Genetic Hallmark: SS18::SSX fusion gene
Synovial sarcomas can present in three distinct histologic variants:
- Monophasic
- Biphasic
- Poorly differentiated
These variants can influence both the diagnostic approach and treatment strategies.
Diagnosis and Challenges
Early and accurate diagnosis of synovial sarcoma is crucial but often challenging due to its rarity and nonspecific symptoms. Patients may initially present with a painless mass or swelling, which can be mistaken for benign conditions. As the tumor grows, it may cause pain, limited mobility, or neurological symptoms if it compresses nearby nerves.
Diagnostic Approach:
- Imaging Studies: X-rays, CT scans, and MRI scans to visualize the tumor and assess its extent
- Biopsy: Surgical removal of tissue for histological examination
- Genetic Testing: Molecular analysis to detect the characteristic SS18::SSX fusion gene
The challenge lies in distinguishing synovial sarcoma from other soft tissue tumors, necessitating expertise in pathology and molecular diagnostics.
What is the standard treatment for synovial sarcoma?
The management of synovial sarcoma typically involves a multidisciplinary approach, combining surgery, radiation therapy, and chemotherapy. The specific treatment plan depends on factors such as tumor size, location, stage, and the patient’s overall health.
1. Surgery
Surgical resection remains the cornerstone of treatment for localized synovial sarcoma. The goal is to achieve complete removal of the tumor with clear margins. In some cases, this may require extensive surgery, potentially including the removal of entire muscle groups or even limb amputation in advanced cases.
2. Radiation Therapy
Radiation therapy is often used in conjunction with surgery to reduce the risk of local recurrence. It may be administered:
- Pre-operatively to shrink the tumor and facilitate surgical removal
- Post-operatively to eliminate any remaining microscopic disease
3. Chemotherapy
The role of chemotherapy in synovial sarcoma treatment remains somewhat controversial, particularly in localized disease. However, it is more commonly used in advanced or metastatic cases. Standard chemotherapy regimens may include:
- Doxorubicin
- Ifosfamide
- High-dose ifosfamide
New Weapons
The limited efficacy of traditional treatments in advanced synovial sarcoma has spurred research into novel therapeutic approaches. Several promising avenues are currently being explored:
- Targeted Therapies
Researchers are investigating drugs that target specific molecular pathways involved in synovial sarcoma growth and progression. For example:
- Pazopanib, a multi-kinase inhibitor, has shown some activity in advanced soft tissue sarcomas, including synovial sarcoma
- Inhibitors of the SS18::SSX fusion protein are in early stages of development
- Immunotherapy
Synovial sarcoma has emerged as a promising candidate for immunotherapy due to its expression of specific antigens:
- NY-ESO-1 Targeted Therapies: NY-ESO-1 is a cancer-testis antigen highly expressed in synovial sarcoma. Clinical trials using NY-ESO-1-directed T cell therapies have shown encouraging results
- Immune Checkpoint Inhibitors: While not as effective as in some other cancer types, ongoing trials are evaluating the potential of checkpoint inhibitors in synovial sarcoma
- Cell-Based Therapies
Adoptive cell therapies, particularly those targeting MAGE-A4 or NY-ESO-1 antigens, have shown promising results in recent clinical trials. These approaches involve modifying a patient’s own T cells to recognize and attack cancer cells expressing specific antigens.
- Combination Approaches
Researchers are exploring various combination strategies to enhance the efficacy of existing treatments:
- Combining targeted therapies with chemotherapy
- Integrating immunotherapy with conventional treatments
- Dual immunotherapy approaches targeting multiple pathways
Prognosis and Gaps in Care
The prognosis for patients with synovial sarcoma varies significantly based on several factors:
- Stage at Diagnosis: Localized disease has a better prognosis than metastatic disease
- Tumor Size: Smaller tumors generally have a more favorable outlook
- Age: Younger patients tend to have better outcomes
- Completeness of Surgical Resection: Achieving clear margins is associated with improved survival
5-Year Survival Rates:
- Localized disease: 60-70%
- Metastatic disease: <20%
Despite advances in treatment, several significant gaps remain in the care of patients with synovial sarcoma:
- Delayed Diagnosis: The rarity of the disease and its nonspecific symptoms often lead to delays in diagnosis, potentially impacting treatment outcomes
- Limited Effective Therapies for Advanced Disease: While surgery can be curative in localized disease, treatment options for metastatic synovial sarcoma remain limited
- Resistance to Conventional Therapies: Many patients develop resistance to standard chemotherapy regimens, highlighting the need for novel approaches
- Long-Term Effects of Treatment: Aggressive treatments can lead to significant long-term morbidity, particularly in young patients
- Access to Specialized Care: The rarity of synovial sarcoma necessitates treatment at specialized sarcoma centers, which may not be accessible to all patients
What Is the Rationale for FDA’s Accelerated Approval of Afami-cel? 
D’Angelo SP, Araujo DM, Abdul Razak AR, et al. Afamitresgene autoleucel for advanced synovial sarcoma and myxoid round cell liposarcoma (SPEARHEAD-1): an international, open-label, phase 2 trial. The Lancet. Published Online March 27, 2024. DOI: https://doi.org/10.1016/S0140-6736(24)00319-2
SPEARHEAD-1 Trial Design and Patient Population
The SPEARHEAD-1 trial was an open-label, non-randomized phase 2 study conducted across 23 sites in Canada, the USA, and Europe.
Key eligibility criteria included:
- HLA-A*02 positive patients
- Advanced (metastatic or unresectable) synovial sarcoma or myxoid round cell liposarcoma
- MAGE-A4 expression in tumor samples
- Previous treatment with anthracycline or ifosfamide-containing chemotherapy
The study enrolled 52 patients (44 with synovial sarcoma, 8 with myxoid round cell liposarcoma) who received afami-cel treatment.
Treatment Protocol
Patients underwent leukapheresis for T-cell collection, followed by afami-cel manufacturing. Prior to infusion, patients received lymphodepletion chemotherapy with fludarabine and cyclophosphamide. Afami-cel was then administered as a single intravenous infusion.
Primary Endpoint and Efficacy Results
The primary endpoint was overall response rate (ORR) as determined by independent review using RECIST v1.1 criteria.
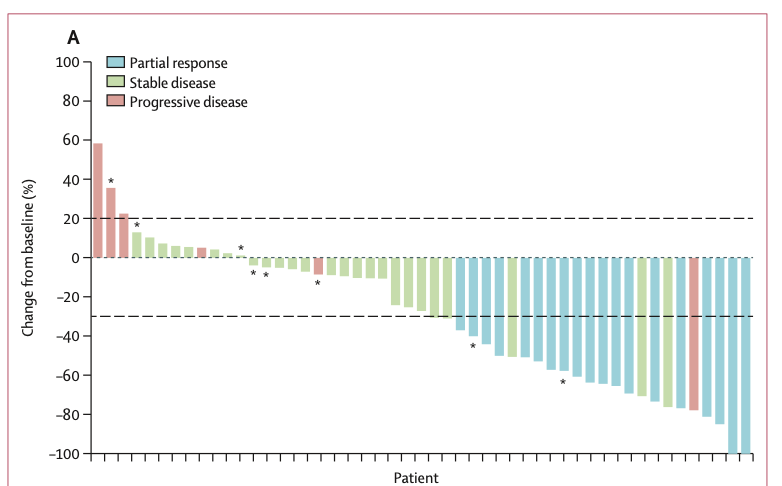
Key efficacy findings include:
- Overall ORR: 37% (19/52 patients, 95% CI: 24-51%)
- Synovial sarcoma ORR: 39% (17/44 patients, 95% CI: 24-55%)
- Myxoid round cell liposarcoma ORR: 25% (2/8 patients, 95% CI: 3-65%)
- Median duration of response: 11.6 months (95% CI: 4.4-18.0) for synovial sarcoma
These results significantly exceeded the pre-specified null hypothesis of 18% ORR, demonstrating the efficacy of afami-cel in this heavily pre-treated patient population.
Secondary Endpoints and Survival Data
- Median time to response: 4.9 weeks (95% CI: 4.3-8.1)
- Median progression-free survival: 3.7 months (95% CI: 2.8-5.6) overall
- Median overall survival: 15.4 months (95% CI: 10.9-28.7)
- 12-month overall survival probability: 60% (95% CI: 46-73%)
Notably, patients with synovial sarcoma who achieved a RECIST response had improved outcomes, with a 24-month overall survival probability of 70% (95% CI: 43-87%).
Safety Profile
The safety profile of afami-cel was generally manageable, with key findings including:
- Cytokine release syndrome (CRS) in 71% of patients, mostly grade 1-2 (one grade 3 event)
- Hematological toxicities, primarily due to lymphodepletion chemotherapy
- No treatment-related deaths or occurrences of replication-competent lentivirus
Biomarker and Translational Insights
The study provided valuable insights into biomarkers and mechanisms of action:
- Higher MAGE-A4 expression correlated with improved response rates
- Increased post-infusion interferon-γ levels associated with CRS severity
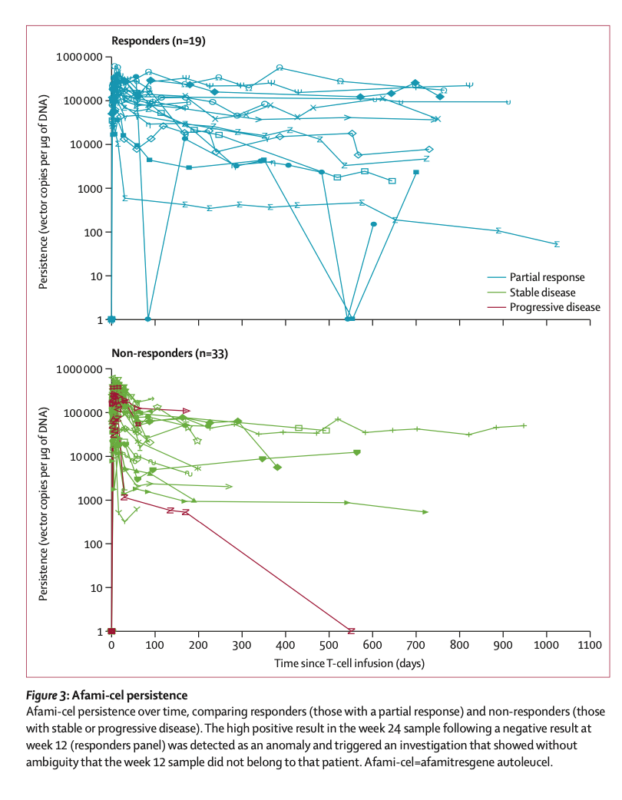
- Exposure-response relationship observed between afami-cel persistence and overall survival
Regulatory Approval and Designations
Afamitresgene autoleucel received accelerated approval from the FDA on August 2, 2024. This approval pathway allows for earlier access to promising therapies for serious or life-threatening conditions based on preliminary evidence of clinical benefit.
What does FDA Accelerated approval mean?
The FDA’s Accelerated Approval Program allows for the earlier approval of drugs that treat serious conditions and address unmet medical needs by using surrogate endpoints, which are markers thought to predict clinical benefit.
This means that drugs can be approved based on preliminary evidence, such as tumor shrinkage in cancer treatments, rather than waiting for long-term clinical outcomes like increased survival rates.
Drug companies benefit from this program as it enables them to bring their products to market more quickly, generating revenue sooner and potentially providing life-saving treatments to patients faster.
However, companies are still required to conduct confirmatory Phase 3 trials to verify the anticipated clinical benefits post-approval. If these trials fail to confirm the benefits, the FDA can withdraw the drug from the market.
The therapy was also granted several expedited designations, including:
- Regenerative Medicine Advanced Therapy (RMAT): Recognizes the potential of the therapy to address unmet medical needs in serious conditions.
- Priority Review: Expedites the review process to bring the therapy to patients sooner.
- Orphan Drug Designation: Provides incentives for the development of therapies for rare diseases.
Conclusion
The FDA approval of afamitresgene autoleucel for synovial sarcoma marks a pivotal moment in solid tumor immunotherapy. The SPEARHEAD-1 trial demonstrated clinically meaningful efficacy and a manageable safety profile in a heavily pre-treated patient population. This breakthrough provides a new treatment option for patients with HLA-A*02-positive, MAGE-A4-expressing synovial sarcoma who have progressed on prior therapies.
The success of afami-cel opens the door for further development of TCR therapies in other solid tumors, potentially revolutionizing the treatment landscape for cancer patients. As research continues, optimizing patient selection, understanding resistance mechanisms, and refining manufacturing processes will be crucial to maximizing the impact of this innovative therapeutic approach.
By demonstrating the feasibility and efficacy of targeting solid tumor antigens with engineered T-cells, the approval of afami-cel represents a significant step forward in the field of cancer immunotherapy. This milestone achievement offers new hope for patients with synovial sarcoma and paves the way for future advancements in the treatment of other challenging solid tumors.