What is immunotherapy?
Immunotherapy is a type of cancer treatment that uses the power of the body’s immune system to fight cancer. It works by stimulating or enhancing the immune system’s ability to recognize and attack cancer cells more effectively. The immune system is a complex network of cells, tissues, and organs that work together to protect the body from foreign invaders like viruses, bacteria, and abnormal cells such as cancer cells. However, cancer cells can sometimes evade or suppress the immune system’s defenses, allowing them to grow and spread unchecked.
Immunotherapy aims to counteract these evasive mechanisms employed by cancer cells and boost the immune system’s ability to detect and eliminate them. It can be achieved through various approaches, including stimulating the body’s immune cells to attack cancer cells more aggressively, providing synthetic immune system proteins to help the immune system function better, or introducing lab-made immune system components to target and destroy cancer cells.
How does immunotherapy work for cancer?
Immunotherapy works by using and enhancing the body’s natural defense mechanisms to fight cancer. There are several mechanisms by which different types of immunotherapies function:
- Checkpoint Inhibitors: These drugs work by blocking specific proteins (checkpoints) on immune cells that normally act as brakes, preventing the immune system from attacking cancer cells. By blocking these checkpoints, the immune cells are unleashed to recognize and attack cancer cells more effectively.
- CTLA-4 inhibitors (e.g., ipilimumab) block the CTLA-4 protein on T cells, preventing it from binding to its ligands and allowing T cells to remain activated to fight cancer. CTLA-4 is an inhibitory receptor that acts as a checkpoint, suppressing the activation and proliferation of T cells.
- PD-1/PD-L1 inhibitors (e.g., pembrolizumab, nivolumab) block the PD-1 protein on T cells or its ligand PD-L1 on cancer cells. This prevents cancer cells from inactivating T cells, allowing the T cells to continue attacking the tumor. PD-1 is another inhibitory receptor on T cells, and its ligand PD-L1 is often expressed by cancer cells as a mechanism to evade immune recognition.
- Monoclonal Antibodies: These are lab-made proteins that can bind to specific targets on cancer cells or immune cells, either marking cancer cells for destruction by the immune system or enhancing the immune response against cancer.
- Some monoclonal antibodies directly bind to tumor-associated antigens on the cancer cell surface, flagging them for destruction by immune cells like natural killer cells or antibody-dependent cell-mediated cytotoxicity (ADCC).
- Bispecific antibodies can simultaneously bind to both a tumor antigen and a receptor on immune cells like T cells, bringing the two cell types together to facilitate tumor killing.
- Cancer Vaccines: These vaccines are designed to stimulate the immune system to recognize and attack specific cancer cells. They work by exposing the immune system to tumor-associated antigens (TAAs), which are substances that can trigger an immune response against the cancer cells expressing those antigens.
- Some vaccines contain tumor antigen peptides or inactivated tumor cells to prime antigen-presenting cells (APCs) like dendritic cells to activate T cells against the tumor.
- Others use vectors like viruses or bacteria to deliver tumor antigens and activate APCs, inducing a targeted immune response against cancer cells expressing those antigens.
- Adoptive Cell Transfer (ACT): In this approach, a patient’s own immune cells (such as T cells) are collected, genetically modified or stimulated to better recognize and attack cancer cells, and then infused back into the patient’s body.
- CAR T-cell therapy involves genetically engineering T cells to express chimeric antigen receptors (CARs) that can bind to specific tumor antigens, enabling them to recognize and kill cancer cells expressing those antigens.
- TIL (Tumor-Infiltrating Lymphocyte) therapy expands tumor-infiltrating lymphocytes that can recognize tumor cells, enhancing their ability to target and destroy cancer cells.
- Cytokines: These are proteins produced by various cells in the body that can stimulate or regulate the immune system. Some cytokines, such as interleukins (e.g., IL-2) and interferons (e.g., IFN-α), can be administered as immunotherapy drugs to boost the immune response against cancer.
- IL-2 stimulates the proliferation and activation of T cells and natural killer cells, enhancing their ability to recognize and kill cancer cells.
- IFN-α can increase the expression of tumor antigens on cancer cells, making them more visible to the immune system, and also has direct anti-tumor effects.
- Oncolytic Virus Therapy: This involves using genetically modified viruses that can selectively infect and kill cancer cells while leaving healthy cells unharmed. The dying cancer cells release tumor-associated antigens, which can stimulate an immune response against the remaining cancer cells.
- Oncolytic viruses are engineered to replicate specifically in cancer cells, causing them to lyse (burst) and release tumor antigens, which can prime the immune system to recognize and attack other cancer cells expressing those antigens.
- Some oncolytic viruses are also engineered to express immune-stimulating molecules, further enhancing the anti-tumor immune response.
These various immunotherapy approaches can be used alone or in combination with other treatment modalities like chemotherapy, radiation therapy, or targeted therapy, depending on the type and stage of cancer, as well as the patient’s individual characteristics and response to treatment.
Discover how CAR T-cell therapy, explained by Dana-Farber Cancer Institute, uses modified T cells from your immune system to target and destroy cancer cells.
What types of cancer can be treated with immunotherapy?
Immunotherapy has shown promise in treating various cancers, with extensive research and approved treatments available for types like melanoma, lung cancer, and certain lymphomas. Clinical trials for these cancers provide specific data on patient outcomes and survival rates. However, for other cancer types, comprehensive data is limited due to fewer approved options, early research stages, challenges in conducting large trials, or variable patient responses, making it difficult to generate clear overarching data.
How long does it take for immunotherapy to work?
The time it takes for immunotherapy to work can vary significantly depending on the type of immunotherapy, the cancer type, and the individual patient’s response. In general, it may take several weeks or even months for immunotherapy to show its full effects.
Some patients may experience an initial period of disease progression or worsening symptoms before the immunotherapy takes effect and the immune system starts attacking the cancer cells effectively. This phenomenon is known as “pseudo-progression” and can be challenging to distinguish from actual disease progression.
For checkpoint inhibitors, which are a commonly used type of immunotherapy, it typically takes several weeks or months for the drugs to stimulate the immune system and produce a measurable response. In clinical trials, the median time to respond to checkpoint inhibitors has ranged from 2 to 6 months.
With adoptive cell therapies like CAR T-cell therapy, the time to response can be relatively shorter, with some patients experiencing a response within a few weeks. However, the durability of the response can vary, and some patients may experience a relapse or resistance over time.
It’s important to note that immunotherapy is not a quick fix, and patience is often required as the treatment works to activate the immune system and mount an effective anti-cancer response. Regular monitoring and follow-up with the healthcare team are essential to assess the treatment’s effectiveness and make any necessary adjustments.
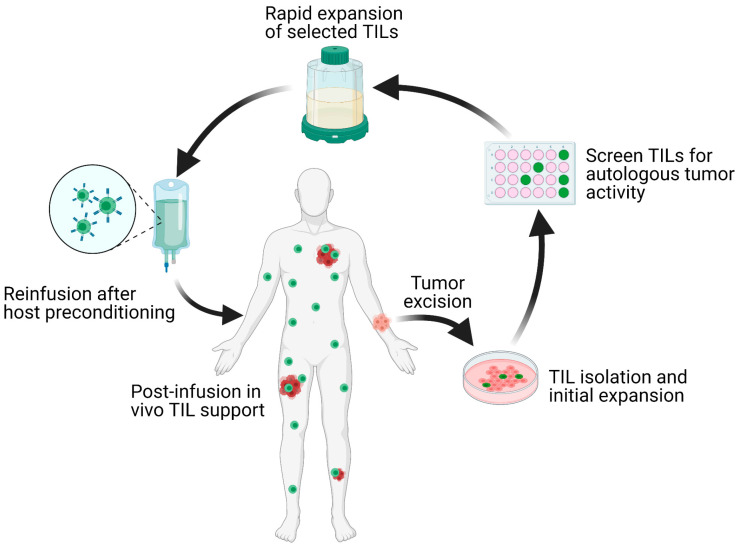
The TIL-ACT protocol isolates and expands tumor-infiltrating lymphocytes (TILs) from metastatic tumor fragments cultured with IL-2, which are then screened for tumor reactivity and rapidly expanded before infusion back into the patient to boost anti-tumor immune responses. The image is taken from the article published by Qin et al (2021).
Why does immunotherapy stop working in some patients?
While immunotherapy has shown remarkable success in treating various cancers, it does not work for all patients, and some patients may experience a relapse or resistance to the treatment over time. There are several potential reasons why immunotherapy may stop working in some patients:
- Tumor Heterogeneity: Cancer cells within a tumor can be genetically diverse, and some subpopulations of cancer cells may develop resistance mechanisms against the specific immunotherapy being used. This heterogeneity can lead to the selection and survival of resistant cancer cell clones that can evade the immune system’s attack.
- Immune Suppression: The tumor microenvironment can become immunosuppressive, preventing the immune cells from effectively recognizing and attacking the cancer cells. This can be due to the presence of immunosuppressive cells like regulatory T cells (Tregs) or myeloid-derived suppressor cells (MDSCs), or the production of immunosuppressive molecules like TGF-β or IL-10.
- Immune Escape: Cancer cells can evolve mechanisms to evade or suppress the immune system’s attack, such as downregulating tumor antigens or expressing inhibitory molecules like PD-L1, which can render immunotherapy ineffective.
- Immune Cell Exhaustion: Prolonged exposure to cancer antigens can lead to the exhaustion of immune cells, rendering them less effective in targeting cancer cells. This can be characterized by the upregulation of inhibitory receptors like PD-1 on T cells and a decreased ability to proliferate and produce cytokines.
- Genetic Alterations: Mutations or genetic changes in cancer cells can alter the expression of target molecules or pathways, making the immunotherapy less effective. For example, mutations in the genes encoding for proteins targeted by checkpoint inhibitors can lead to resistance.
- Microbiome Changes: Alterations in the gut microbiome composition have been linked to reduced efficacy of certain immunotherapies, potentially due to changes in immune regulation and the tumor microenvironment.
To overcome resistance and improve the durability of immunotherapy responses, researchers are exploring combination strategies, such as combining different immunotherapies or combining immunotherapy with other treatment modalities like chemotherapy, targeted therapy, or radiation therapy. Additionally, ongoing research aims to identify predictive biomarkers and develop personalized approaches to immunotherapy based on the individual patient’s tumor characteristics and immune profile.
Am I a good candidate for immunotherapy?
Determining whether you are a good candidate for immunotherapy depends on various factors, including the type and stage of your cancer, your overall health status, and the availability of approved immunotherapies for your specific cancer type. It is crucial to discuss your individual circumstances with your oncologist or healthcare team, who can evaluate your medical history, cancer characteristics, and potential risks and benefits of immunotherapy.
Certain tests, such as biomarker testing or genetic profiling, may be required to determine if you are likely to respond to a particular immunotherapy. For example, some immunotherapies are more effective in cancers with high levels of specific biomarkers like PD-L1 expression or a high tumor mutational burden. Your doctor may recommend these tests to assess your eligibility and the potential effectiveness of different immunotherapy options.
It’s important to note that immunotherapy is not suitable for all patients, and factors such as overall health, immune system function, and the presence of certain autoimmune disorders may affect your eligibility or increase the risk of side effects. Additionally, your cancer type, stage, and previous treatments may also influence whether immunotherapy is an appropriate choice for you.
Ultimately, the decision to pursue immunotherapy should be made in consultation with your healthcare team, taking into account the potential benefits and risks, as well as your individual preferences and circumstances. Open communication with your doctor and undergoing any necessary tests are crucial steps in determining if you are a good candidate for immunotherapy
Sources
- Adoptive T Cell Therapy for Solid Tumors: Pathway to Personalized Standard of Care – Cells
- How Immunotherapy Is Used to Treat Cancer – American Cancer Society
- Immunotherapy to Treat Cancer – National Cancer Institute
- A guide to cancer immunotherapy: from T cell basic science to clinical practice – Nature Reviews Immunology
- A review of cancer immunotherapy: from the past to the present, to the future – Current Oncology
- Clinical cancer immunotherapy: Current progress and prospects – Frontiers in Immunology
- Combining Oncolytic Viruses With Cancer Immunotherapy: Establishing a New Generation of Cancer Treatment – Frontiers in Immunology
- Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy – Cell
- Types of cancer immunotherapy – Cancer Research UK
- Pembrolizumab plus Chemotherapy in Advanced Triple-Negative Breast Cancer – NEJM
- Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma – NEJM
- OncoDaily


