Breakthrough Emactuzumab Phase 3 TANGENT Trial in TGCT: SynOx Pharma Reports Enrollment Completed Ahead of Schedule
On August 5, 2025, SynOx Therapeutics Limited today announced the completion of patient enrollment in the registrational Phase 3 TANGENT trial evaluating emactuzumab in patients with Tenosynovial Giant Cell Tumor (TGCT) not amenable to surgery. Enrollment concluded significantly ahead of schedule, with top-line results expected in the first quarter of 2026.
“Completion of enrollment in our registrational TANGENT trial marks an important milestone for SynOx and for the TGCT community”
said Elyse Seltzer, M.D., Chief Medical Officer of SynOx Therapeutics.
“We are extremely grateful to the patients, families, investigators, and clinical sites whose dedication made this achievement possible and allowed us to enroll the study well ahead of schedule. Subject to data, we remain committed to providing a much-needed new treatment option for this challenging condition.”

LinkedIn photo of Elyse Seltzer
Ray Barlow, Chief Executive Officer of SynOx, said,
“This is an exciting and transformative time for SynOx as we move emactuzumab through its pivotal Phase 3 program. We are enthusiastic about the upcoming steps and look forward to top-line results that we hope will highlight emactuzumab’s potential to significantly improve care for patients affected by this serious disease.”
LinkedIn photo of Ray Barlow
About the TANGENT Trial
The Phase 3 TANGENT study of emactuzumab in tenosynovial giant cell tumor (TGCT) was highlighted at the 2025 ASCO Annual Meeting and published in the Journal of Clinical Oncology (JCO 2025; Vol 43, Suppl 16). The abstract was co-authored by Hans Gelderblom, Emanuela Palmerini, Jean-Yves Blay, and colleagues. The Phase 3 TANGENT trial (NCT05417789) is a randomized, double-blind, placebo-controlled study designed to confirm its efficacy and safety in patients not eligible for surgery.
In Part 1, participants will be randomized in a 2:1 ratio to receive either emactuzumab 1000 mg or placebo intravenously every two weeks for five doses over 10 weeks, followed by a three-month observation period.
- Primary endpoint: Objective Response Rate (ORR) by RECIST criteria at six months.
- Key secondary endpoints: patient-reported outcomes (PROMIS-PF), joint function (range of motion, pain, stiffness), durability of response, and safety.
Part 2 extends from six months to two years post-randomization, allowing patients whose disease progresses to receive open-label emactuzumab.
FDA Grants Fast Track Designation to Emactuzumab in TGCT
In April 2025, the U.S. Food and Drug Administration (FDA) granted Fast Track Designation to emactuzumab, a CSF-1R–targeting monoclonal antibody, for the treatment of patients with unresectable tenosynovial giant cell tumor (TGCT). The designation underscores the significant unmet medical need for effective therapies in patients for whom surgery is not feasible or unlikely to provide meaningful benefit.
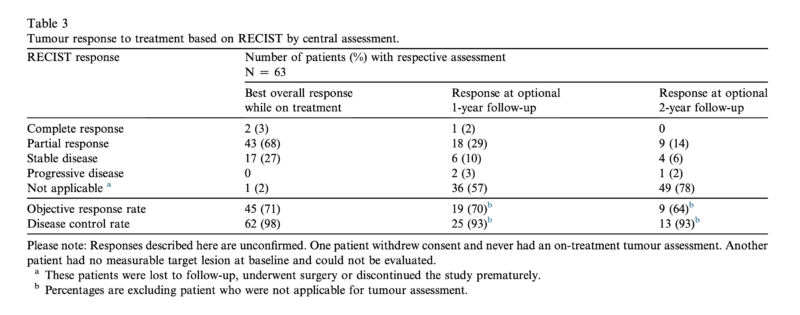
The decision is supported by data from a phase 1 clinical trial (NCT01494688), which results were published in EJC 2020. In the study, 63 patients received emactuzumab at different dosing schedules, with the optimal biological dose identified as 1000 mg intravenously. Patients typically underwent four to five cycles of treatment. The therapy showed strong anti-tumor activity and durable responses.
Key efficacy results:
- 71% of patients experienced measurable tumor shrinkage.
- 98% of patients had at least stable disease.
- Durable responses: 70% maintained tumor reduction at one year, and 64% at two years.
- Rapid onset: 86% showed metabolic responses on FDG-PET scans within one month.
- Retreatment after disease progression could lead to renewed partial responses.
Biomarker analyses showed significant reductions in CSF1R-positive macrophages and CD68/CD163-positive cells in the tumor environment, confirming that emactuzumab effectively targets the cells driving tumor growth. Patients also reported meaningful improvements in quality of life, including better joint function, reduced pain, and overall well-being, as measured by EQ-5D-3L and WOMAC scores.
The treatment was generally well tolerated. Most adverse events were mild to moderate, with common side effects including itching (pruritus), fatigue (asthenia), and swelling (edema). Importantly, no treatment-related deaths occurred during the study.
What is Emactuzumab and How does it Work?
Emactuzumab is a humanized IgG1 monoclonal antibody developed by SynOx Therapeutics (originally in-licensed from Roche) that selectively targets the colony-stimulating factor 1 receptor (CSF-1R). The drug is designed to address the underlying biology of tenosynovial giant cell tumor (TGCT), a disease driven by overproduction of CSF-1, which recruits and maintains an abnormal population of tumor-associated macrophages (TAMs) in the synovial tissue. These macrophages contribute to joint inflammation, tumor growth, and functional impairment.
By binding to CSF-1R, emactuzumab blocks CSF-1 signaling, leading to the depletion of TAMs in the tumor microenvironment. This targeted immune modulation results in:
- Tumor shrinkage, by removing the macrophage support that drives growth.
- Symptom relief, including reduced pain, stiffness, and improved joint mobility.
- Durable responses, as the disruption of TAM-driven pathology addresses a root cause of disease progression.
Unlike conventional therapies that target tumor cells directly, emactuzumab works by modifying the tumor microenvironment, highlighting its potential as a precision therapy for macrophage-driven disorders such as TGCT.
What is Tenosynovial Giant Cell Tumor?
Tenosynovial Giant Cell Tumor (TGCT) is a rare, usually benign tumor affecting the synovium, the tissue lining joints and tendons. It arises from excessive proliferation of synovial cells and is driven by a chromosomal translocation that overproduces colony-stimulating factor-1 (CSF-1), recruiting macrophages to the tumor. TGCT is classified into two forms:
- Localized TGCT (Giant Cell Tumor of Tendon Sheath): Often a slow-growing, painless mass, typically in the fingers or hands. It is the second most common hand tumor after ganglion cysts.
- Diffuse TGCT (Pigmented Villonodular Synovitis – PVNS): Less common but more aggressive, usually affecting larger joints like the knee. It can cause swelling, pain, stiffness, and, if untreated, joint damage.
Although benign, TGCT can severely limit joint function and mobility. It mostly affects adults aged 25–50, with slightly higher incidence of localized TGCT in females. The global incidence is estimated at 43 cases per 1 million people, with localized TGCT being more frequent.
Symptoms typically include pain, swelling, tenderness, and joint instability. Diagnosis often requires imaging, such as X-rays or MRI, and sometimes a biopsy to distinguish TGCT from other joint conditions.
Treatment Options for TGCT
Surgery is the standard treatment for TGCT, but recurrence is common, particularly in diffuse cases. For patients who are not surgical candidates or experience relapse, targeted therapies offer alternatives. These include pexidartinib (Turalio), imatinib (Gleevec), and more recently, Vimseltinib (Romvimza). Vimseltinib has primarily been studied as a monotherapy in TGCT and some advanced solid tumors, showing promising efficacy and tolerability in early trials. Ongoing research, including studies like MOTION, is evaluating its safety, long-term outcomes, and potential future combinations with other therapies.
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023

