
Intravesical Chemotherapy for Non-Muscle Invasive Bladder Cancer: Agents, Strategies, Resistance, and Future Innovations
Bladder cancer is one of the most common malignancies of the urinary tract, with the majority of cases presenting as non-muscle invasive bladder cancer (NMIBC) at diagnosis. NMIBC is confined to the mucosa or submucosa, yet it is characterized by a high risk of recurrence and potential progression. Given the bladder’s unique anatomy as a hollow, self-contained organ, it provides an ideal setting for localized drug delivery. Intravesical chemotherapy exploits this advantage by allowing direct exposure of the urothelium to therapeutic agents, thereby minimizing systemic toxicity while achieving high local drug concentrations. As part of the standard-of-care following transurethral resection of bladder tumor (TURBT), intravesical therapy plays a critical role in reducing recurrence and delaying progression—particularly in patients for whom bladder preservation is a priority.
With growing interest in bladder-sparing strategies, this article explores the mechanisms, commonly used agents, delivery techniques, therapeutic outcomes, resistance challenges, recent innovations, and emerging clinical trials shaping the future of intravesical chemotherapy.
Rationale for Intravesical Chemotherapy: Targeting Superficial Bladder Tumors Through Localized Drug Delivery
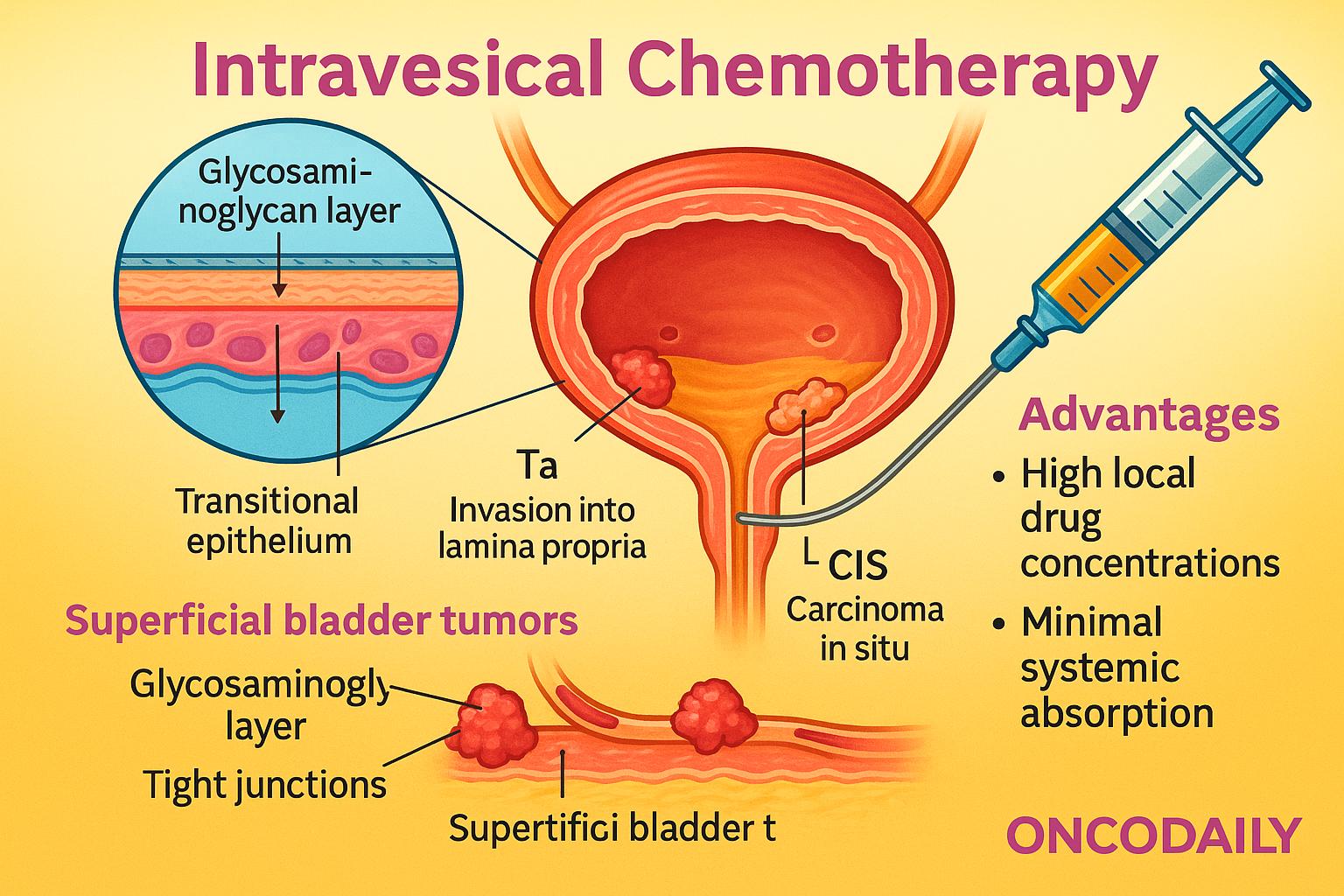
The urothelium, a specialized transitional epithelium lining the bladder, serves as a highly impermeable barrier designed to protect underlying tissues from urine and toxins. This barrier function is maintained by tight junctions, a glycosaminoglycan layer, and low cellular turnover, all of which contribute to limited passive drug absorption. While these features pose a challenge for drug penetration, they also enhance drug retention within the bladder lumen, allowing prolonged contact between therapeutic agents and the tumor surface.
Superficial bladder tumors—particularly those classified as non-muscle invasive (Ta: non-invasive papillary carcinoma, T1: invasion into lamina propria, and CIS: carcinoma in situ)—are confined to the inner layers of the bladder wall. This anatomical accessibility makes them ideal targets for intravesical chemotherapy, as drugs can directly reach the tumor site without needing to penetrate deep tissues.
Compared to systemic chemotherapy, intravesical administration offers significant advantages. It allows high local drug concentrations with minimal systemic absorption, thereby reducing the risk of systemic side effects such as myelosuppression or gastrointestinal toxicity. This local approach is especially beneficial in NMIBC, where disease is limited to the urothelium and early intervention can prevent progression to muscle-invasive stages.
Intravesical Chemotherapeutic Agents: Classes, Mechanisms, and Emerging Strategies
- Alkylating Agents: Mitomycin C is the most widely used alkylating agent for intravesical chemotherapy in NMIBC. Its mechanism involves DNA crosslinking, leading to inhibition of DNA synthesis and tumor cell apoptosis. Mitomycin C is favored for its stability in urine and minimal systemic absorption. Pharmacokinetically, it exhibits effective drug-tissue interaction within short dwell times, typically administered weekly for six weeks as induction, with maintenance schedules in select patients. Clinical trials have demonstrated its efficacy in reducing recurrence rates, particularly in low- to intermediate-risk NMIBC. However, resistance can develop through mechanisms such as increased DNA repair activity or reduced intracellular drug accumulation. Strategies to overcome resistance include combining Mitomycin C with agents that modulate permeability (e.g., electromotive drug administration) or alternating it with other intravesical therapies.
- Anthracyclines: Doxorubicin and Epirubicin are anthracycline antibiotics used intravesically for their DNA intercalating and topoisomerase II inhibitory actions. They are characterized by rapid cellular uptake and strong cytotoxicity but may have lower efficacy compared to Mitomycin C in long-term recurrence prevention. Pharmacologically, both agents exhibit adequate urothelial penetration and urinary stability, though Epirubicin is generally better tolerated. Their use is often limited by chemical cystitis and color-related urine discoloration, but they remain effective alternatives in cases of Mitomycin intolerance or intermediate-risk disease.
- Taxanes and Other Novel Agents: Docetaxel and Gemcitabine represent newer intravesical options, particularly in patients who are refractory or intolerant to Bacillus Calmette-Guérin (BCG) therapy. Docetaxel stabilizes microtubules and inhibits cell division, while Gemcitabine inhibits DNA synthesis via nucleotide analog action. Both drugs have demonstrated favorable tolerability and promising efficacy in recurrent NMIBC, especially in BCG-unresponsive populations. Their solubility profiles and minimal urothelial irritation make them suitable for repeated instillations, and ongoing studies continue to define their role as salvage or frontline agents in selected cohorts.
- Combinatorial and Sequential Regimens: To enhance efficacy and prevent recurrence, several clinical trials have explored combination and sequential intravesical regimens. For example, alternating Mitomycin C and BCG, or using Gemcitabine followed by Docetaxel, has shown synergistic effects with reduced recurrence rates. Such regimens aim to exploit complementary mechanisms of action and reduce the risk of resistance development. Emerging protocols increasingly tailor drug selection and scheduling based on tumor risk stratification and previous treatment response, marking a shift toward personalized intravesical chemotherapy strategies.
BCG Immunotherapy: The Cornerstone of Intravesical Treatment for High-Risk NMIBC
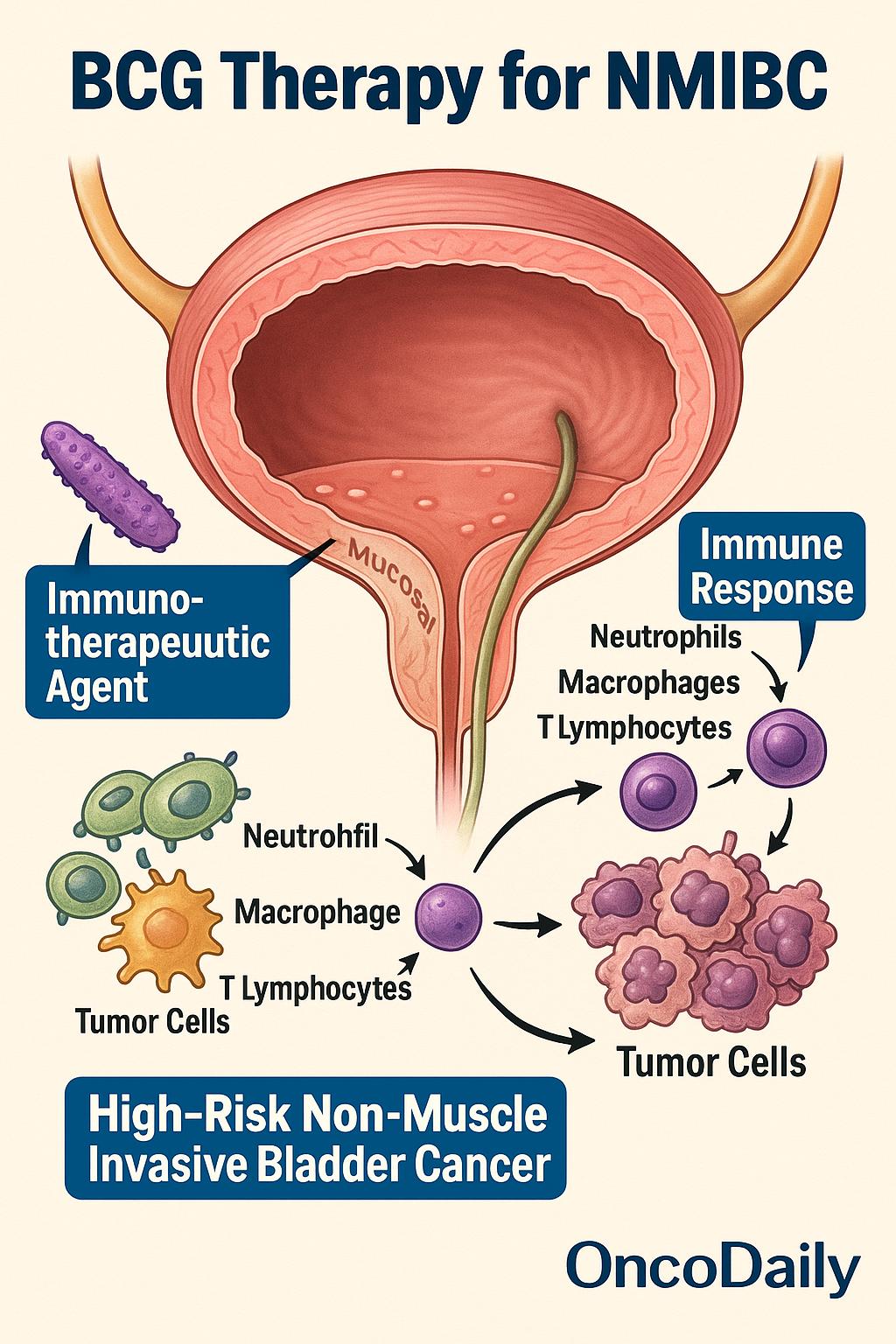
Bacillus Calmette-Guérin (BCG) represents the most effective intravesical therapy for high-risk non-muscle invasive bladder cancer (NMIBC), including carcinoma in situ (CIS), high-grade Ta tumors, and T1 lesions. Unlike traditional chemotherapeutic agents, BCG functions as an immunotherapeutic agent. When instilled into the bladder, it stimulates a robust local immune response, recruiting neutrophils, macrophages, and T lymphocytes that collectively mediate tumor cell cytotoxicity. The antitumor effect is driven by cytokine release—particularly interleukin-2, interferon-γ, and TNF-α—which enhances both innate and adaptive immunity within the bladder wall.
BCG is considered first-line treatment for high-risk NMIBC following complete transurethral resection (TURBT), with extensive evidence supporting its use in both induction (weekly instillations for 6 weeks) and maintenance regimens(typically over 1–3 years). Randomized trials and meta-analyses have consistently shown that BCG reduces recurrence, delays progression, and lowers the risk of radical cystectomy compared to chemotherapy alone.
However, BCG is not without challenges. Local adverse effects such as dysuria, frequency, and hematuria are common, while systemic complications, including fever and rare cases of disseminated BCG infection, may limit its use. Moreover, BCG intolerance and global supply shortages have further complicated its availability and application. A significant clinical dilemma arises in BCG-unresponsive disease, where persistent or recurrent tumors despite adequate BCG exposure necessitate alternative strategies.
In recent years, salvage intravesical therapies—including Gemcitabine, Docetaxel, and sequential doublet regimens—have shown promise in this setting. Additionally, multiple ongoing clinical trials are evaluating novel agents, such as immune checkpoint inhibitors and gene-based therapies, to address the unmet need in BCG-failure patients. As the therapeutic landscape evolves, BCG remains a benchmark against which emerging modalities are measured.
Administration Strategies and Delivery Innovations in Intravesical Chemotherapy
Effective intravesical chemotherapy depends not only on the choice of agent but also on precise delivery techniques that maximize drug contact with the urothelium. Catheterization remains the standard method for drug instillation, typically using a sterile, lubricated catheter inserted transurethrally into the bladder. Once the drug is instilled, the catheter is removed, and patients are instructed to retain the solution for a defined dwell time, usually ranging from 1 to 2 hours, during which position changes may be recommended to ensure even distribution across the bladder wall. Afterward, the bladder is drained, and patients are encouraged to void completely.
Dosing schedules vary depending on the clinical context. According to EAU, AUA, and NCCN guidelines, an induction course consists of weekly instillations for 6 weeks, while maintenance therapy—critical in high-risk NMIBC—may involve monthly or every-3-month treatments for up to 1 to 3 years. Maintenance has been shown to reduce long-term recurrence and progression, particularly when using BCG or Mitomycin C.
To enhance the efficacy and penetration of intravesical agents, several physical and chemical delivery adjuncts have been developed. Electromotive drug administration (EMDA) uses electric current to drive charged drug molecules across the urothelium, improving tissue uptake. Hyperthermia-assisted delivery, which heats the drug solution or bladder wall, enhances cytotoxicity and drug diffusion by increasing membrane permeability. Similarly, nanocarriers—such as liposomes or polymeric nanoparticles—are being explored to improve drug stability and targeted delivery. Biodegradable implants that release drugs over extended periods directly into the bladder lumen are also under investigation, offering a promising strategy for overcoming challenges of patient compliance and frequent dosing.
Clinical Efficacy of Intravesical Chemotherapy in NMIBC: Insights from Trials and Meta-Analyses
Intravesical chemotherapy remains a cornerstone in the management of non-muscle invasive bladder cancer (NMIBC), particularly following transurethral resection (TURBT). Evidence consistently supports its role in reducing recurrence, with evolving data also informing its impact on progression and the need for radical cystectomy.
Recent pooled analyses underscore the efficacy of mitomycin C (MMC) and gemcitabine in improving recurrence-free survival (RFS). A 2023 meta-analysis involving 49 studies reported pooled 1-year RFS rates of 67.2% for MMC and 69.5% for gemcitabine, with relative risk reductions in recurrence of 37% and 24%, respectively (PMC11674742). While progression data are less uniform, MMC induction plus maintenance therapy has demonstrated 1- and 2-year RFS rates of 84% and 75% in intermediate-risk populations—comparable to those seen with BCG.
BCG continues to outperform chemotherapy in high-risk NMIBC. Multiple randomized controlled trials and systematic reviews have established its superiority over MMC in reducing both recurrence and progression, especially when used with a maintenance schedule. However, for intermediate-risk NMIBC, MMC with maintenance achieves near-equivalent outcomes and serves as a viable alternative, particularly in the context of BCG shortages or intolerance.
Immediate post-operative instillation of MMC following TURBT has also demonstrated clear benefit. In low-risk patients, a single dose reduces 5-year cumulative recurrence by up to 40%, reinforcing its role in frontline management as endorsed by the EAU, AUA, and NCCN guidelines.
Stratification by risk remains critical in guiding therapeutic selection:
-
Low-risk NMIBC benefits from TURBT followed by immediate single-dose MMC.
-
Intermediate-risk patients show excellent outcomes with either MMC or BCG, provided maintenance is included.
-
High-risk NMIBC, including CIS and high-grade Ta/T1 lesions, is best managed with intravesical BCG, which offers superior protection against both recurrence and disease progression.
While BCG remains the benchmark, advances in intravesical drug delivery and growing evidence for agents like gemcitabine are expanding the therapeutic landscape—particularly for patients with BCG-unresponsive disease or limited access to immunotherapy. Ongoing trials will further clarify the optimal sequencing and combinations in these settings.
Mechanisms of Resistance to Intravesical Chemotherapy: Biological Barriers and Emerging Biomarkers
Despite the effectiveness of intravesical chemotherapy in reducing recurrence in NMIBC, a significant subset of patients eventually experiences disease recurrence or progression. Resistance to therapy is driven by a combination of tumor-intrinsic factors and microenvironmental barriers that limit drug efficacy.
One of the most studied resistance mechanisms involves drug efflux pumps, particularly ATP-binding cassette (ABC) transporters such as P-glycoprotein (ABCB1). These proteins actively export chemotherapeutic agents like mitomycin C and doxorubicin from urothelial cells, reducing intracellular drug accumulation and diminishing cytotoxic effects. Overexpression of these transporters has been associated with treatment failure in multiple studies.
Enhanced DNA repair capacity represents another key mechanism. Tumor cells that overexpress nucleotide excision repair (NER) enzymes or have upregulated homologous recombination pathways can efficiently repair the DNA damage caused by alkylating agents or anthracyclines. This allows cancer cells to survive cytotoxic injury and promotes clonal persistence or regrowth.
Increased epithelial turnover and urothelial shedding may also compromise drug exposure. Rapid regeneration of the urothelium can limit the duration of drug contact with malignant cells, especially in the context of superficial papillary tumors. Moreover, the inherent heterogeneity of tumor biology—including variations in proliferation rates, molecular subtypes, and immune infiltration—modulates response to therapy and risk of recurrence.
The correlation between recurrence risk and tumor biology is further supported by clinical data. High-grade and multifocal tumors, as well as those with CIS or early recurrence after induction therapy, tend to display molecular features linked to resistance, such as TP53 mutations, FGFR3 alterations, and high proliferative indices. Inadequate drug exposure—whether due to suboptimal dwell time, impaired retention, or poor compliance—also significantly increases the likelihood of recurrence, particularly in intermediate- and high-risk groups.
Several biomarkers are currently under investigation to predict response or resistance to intravesical therapy. Promising candidates include:
-
MDR1 gene expression (encoding P-glycoprotein)
-
ERCC1 and RAD51 (DNA repair proteins)
-
Ki-67 (proliferation marker)
-
miRNAs, such as miR-21 and miR-200c, implicated in chemotherapy sensitivity
While none have yet entered routine clinical use, ongoing prospective studies aim to integrate molecular profiling into risk-adapted treatment algorithms. The identification of validated predictive biomarkers will be essential for advancing personalized intravesical therapy in NMIBC.
Safety Profile and Toxicity Management in Intravesical Chemotherapy
Intravesical chemotherapy is generally well tolerated, particularly when compared to systemic therapy. However, local adverse effects are relatively common and can impact patient compliance, particularly in long-term maintenance protocols.
The most frequent local toxicities include dysuria, chemical cystitis, and hematuria. These symptoms typically arise due to the irritative nature of chemotherapeutic agents on the bladder mucosa and may present as urinary frequency, urgency, burning sensation, or visible blood in the urine. Mitomycin C and epirubicin are more commonly associated with these side effects, especially when instillation intervals are frequent or when dosing volumes are high.
Systemic absorption of intravesical agents is rare due to the urothelium’s barrier properties, but when it occurs—particularly in the context of mucosal disruption, inflammation, or catheter-induced trauma—it may result in systemic toxicity. Documented cases include neutropenia or gastrointestinal symptoms with mitomycin C and flu-like symptoms with epirubicin. These events are infrequent but warrant vigilance, especially in elderly or frail patients.
Over time, repeated instillations may lead to chronic bladder changes, including fibrosis, reduced compliance, and decreased bladder capacity. These changes are more likely in patients receiving long-term maintenance therapy or those with baseline urothelial inflammation. Although rare, such complications can significantly affect quality of life and may necessitate discontinuation of therapy or surgical intervention.
Several strategies are employed to mitigate toxicity:
-
Dose adjustments based on patient tolerance and comorbidities are essential, particularly for those with small bladder volumes or pre-existing urinary symptoms.
-
Pre- and post-instillation hydration helps dilute urinary concentrations of the drug and flush residual irritants, reducing mucosal exposure.
-
Post-instillation protocols, including encouraging patients to avoid voiding for 1–2 hours and rotating body position during dwell time, may enhance distribution and minimize focal irritation.
-
Use of uroprotective agents (e.g., sodium hyaluronate) is under investigation but not yet standard practice.
Careful patient selection, monitoring, and supportive care can significantly reduce the incidence and severity of adverse events, allowing patients to complete full therapeutic courses and derive maximal benefit from intravesical chemotherapy.
Innovations and Future Directions in Intravesical Chemotherapy
The field of intravesical chemotherapy is undergoing rapid transformation, driven by innovations in drug delivery technologies and combination strategies that aim to overcome longstanding limitations such as poor tissue penetration, frequent dosing, and drug resistance.
One promising avenue is the development of nanoparticle-based formulations, which enhance drug solubility, stability, and controlled release. Nanocarriers—such as liposomes, polymeric nanoparticles, and micelles—can improve urothelial uptake and prolong mucosal contact time. Preclinical studies have demonstrated increased efficacy and reduced toxicity with nanoparticle-encapsulated mitomycin C and doxorubicin, while early-phase trials are investigating their translational potential in NMIBC.
Sustained-release gels represent another innovation in intravesical drug retention. A notable example is UGN-101(mitomycin gel), a reverse-thermal gel that solidifies at body temperature, allowing prolonged exposure to the urothelium. Approved by the FDA for low-grade upper tract urothelial carcinoma (UTUC), this platform is now being explored for NMIBC applications. Similarly, implantable drug-eluting devices are under development to provide continuous, long-term intravesical delivery without the need for repeated catheterizations, potentially improving adherence and quality of life.
Advances in targeted delivery systems are also gaining traction. Ligand-conjugated nanoparticles designed to bind urothelial or tumor-specific markers (e.g., integrins or EGFR) offer the promise of selective cytotoxicity with minimal off-target effects. Although still largely in the experimental phase, these approaches may enable personalized and precision-based intravesical therapy in the near future.
A particularly exciting area is the exploration of immuno-chemotherapy combinations. With the increasing understanding of the tumor immune microenvironment, clinical trials are evaluating the synergy between intravesical chemotherapy and immune checkpoint inhibitors. Early data suggest that combining agents such as gemcitabine with anti-PD-1/PD-L1 therapy may enhance immunogenic cell death and improve durable responses in BCG-unresponsive NMIBC.
Several ongoing clinical trials are actively shaping the future of intravesical therapy:
-
NCT04164082: Phase II study of sequential gemcitabine/docetaxel in BCG-unresponsive NMIBC.
-
NCT03081858: Evaluating intravesical gemcitabine with pembrolizumab.
-
NCT04730219: Investigating sustained-release hydrogel-based intravesical mitomycin in intermediate-risk NMIBC.
-
NCT02773849 (KEYNOTE-676): A Phase III trial of pembrolizumab with or without chemotherapy for BCG-unresponsive disease.
These studies reflect a broader shift toward mechanism-driven, individualized therapies, integrating drug delivery science with immuno-oncology and molecular profiling. As these innovations progress from bench to bedside, they hold the potential to expand treatment options, prolong bladder preservation, and improve outcomes in patients with NMIBC.
You Can Watch More on OncoDaily Youtube TV
Written by Toma Oganezova, MD
FAQ
What is intravesical chemotherapy in bladder cancer?
It’s a localized treatment where chemotherapy is directly instilled into the bladder to treat NMIBC and prevent recurrence.
When is intravesical chemotherapy used?
It’s used after TURBT in non-muscle invasive bladder cancer (NMIBC) to reduce recurrence and progression.
Which drugs are commonly used for intravesical therapy?
Mitomycin C, gemcitabine, doxorubicin, epirubicin, and docetaxel are among the most frequently used agents.
What is BCG therapy and how does it differ?
BCG is an intravesical immunotherapy used mainly for high-risk NMIBC, stimulating immune response rather than direct cytotoxicity.
Is intravesical chemotherapy better than systemic chemo?
For early-stage NMIBC, intravesical therapy is preferred due to high local effect and fewer systemic side effects.
What are common side effects of intravesical chemotherapy?
Dysuria, cystitis, hematuria, and rarely systemic absorption causing fatigue or neutropenia.
Can BCG-unresponsive patients benefit from intravesical chemotherapy?
Yes, agents like gemcitabine and docetaxel are used in BCG-unresponsive NMIBC as salvage options.
What is EMDA and how does it enhance therapy?
Electromotive Drug Administration (EMDA) uses electric current to enhance drug penetration across the bladder wall.
Are new delivery methods being developed?
Yes, nanocarriers, reverse-thermal gels (e.g., UGN-101), and drug-eluting devices are under investigation.
What clinical trials are shaping future intravesical therapy?
Trials like KEYNOTE-676 and NCT04164082 are evaluating chemo-immunotherapy and sustained-release systems for NMIBC.
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
