Common Side Effects of Oral Chemotherapy and How to Manage Them
Oral chemotherapy has revolutionized the landscape of cancer treatment by offering patients convenience, reduced hospital visits, and greater autonomy. These agents—ranging from traditional cytotoxics to targeted therapies—are increasingly used across cancer types including breast, colorectal, lung, and hematologic malignancies. Despite their benefits, oral chemotherapy is associated with significant side effects, which may differ in spectrum, onset, and management from intravenous regimens.
This article reviews the mechanisms behind these side effects, their clinical manifestations, and current strategies for mitigating toxicity while maintaining treatment efficacy.
Classification of Oral Chemotherapy Agents and Their Clinical Applications
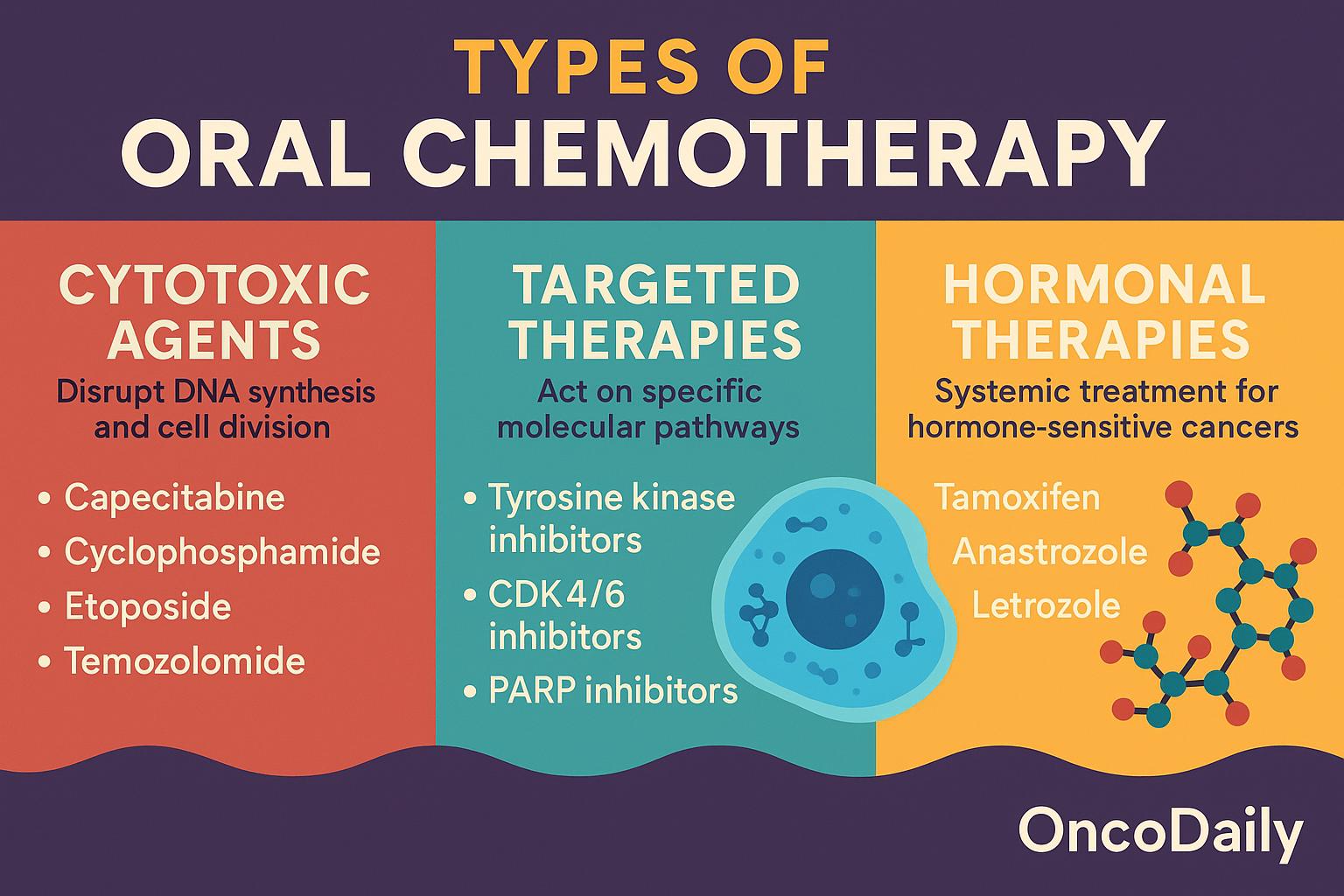
Oral chemotherapy encompasses a broad spectrum of agents with diverse mechanisms of action, pharmacokinetic profiles, and toxicity patterns. These drugs can be broadly classified into three categories: cytotoxic agents, targeted therapies, and hormonal therapies. Although not all are classical chemotherapeutics, their oral route and systemic anticancer activity warrant inclusion in this discussion. Understanding their classification is essential to contextualize their distinct side effect profiles.
Cytotoxic Oral Chemotherapy Agents
Cytotoxic agents act by disrupting DNA synthesis, cell division, or mitotic spindle formation, targeting rapidly dividing cells indiscriminately. Several of these traditional agents have been reformulated or developed as oral drugs to improve convenience and outpatient administration.
- Capecitabine is a prodrug of 5-fluorouracil (5-FU) that undergoes enzymatic conversion in tumor tissues. It is used in colorectal, breast, and gastric cancers.
- Cyclophosphamide, an alkylating agent, exerts its cytotoxicity by forming DNA cross-links and is used in breast cancer, lymphomas, and autoimmune malignancies.
- Etoposide, a topoisomerase II inhibitor, induces DNA strand breaks and is used for small cell lung cancer and testicular cancer.
- Temozolomide is an oral alkylating agent that crosses the blood-brain barrier and is primarily used in glioblastoma and anaplastic astrocytoma.
These agents are associated with classic chemotherapy toxicities such as myelosuppression, mucositis, gastrointestinal disturbances, and alopecia, due to their non-selective activity against proliferating cells.
Targeted Oral Therapies
Targeted therapies act on specific molecular pathways involved in cancer cell proliferation and survival. Their increased specificity often translates to a distinct toxicity profile, although off-tumor, on-target effects are still common.
- Tyrosine kinase inhibitors (TKIs), such as imatinib, osimertinib, and erlotinib, inhibit key signaling pathways like BCR-ABL, EGFR, and ALK. These are indicated in chronic myeloid leukemia (CML), non-small cell lung cancer (NSCLC), and gastrointestinal stromal tumors (GISTs).
- CDK4/6 inhibitors (e.g., palbociclib, ribociclib) interfere with cell cycle progression and are mainly used in hormone receptor–positive, HER2-negative breast cancer.
- PARP inhibitors, including olaparib and niraparib, impair DNA repair in BRCA-mutated cells and are approved for ovarian, breast, pancreatic, and prostate cancers.
While these agents often spare normal proliferating tissues, they introduce new toxicity patterns—such as dermatologic reactions, QT prolongation, diarrhea, and hepatotoxicity—often linked to the pathways they target in normal tissues.
Hormonal Therapies with Chemotherapy-Like Impact
Although not chemotherapeutic in the classical sense, several oral hormonal agents are used as first-line systemic therapies in hormone-sensitive malignancies. These include tamoxifen, a selective estrogen receptor modulator (SERM), and aromatase inhibitors like anastrozole and letrozole. These agents are standard treatments in breast and endometrial cancers.
Their side effects are largely hormonal and metabolic in nature, such as hot flashes, arthralgia, thromboembolic events, and osteoporosis. Nonetheless, their long-term systemic impact and frequent use alongside or after chemotherapy justify their inclusion in comprehensive toxicity evaluations.
Mechanistic Basis of Oral Chemotherapy Toxicities
The side effects of oral chemotherapy are not random but arise from well-defined biological mechanisms. Understanding the pathophysiology behind these toxicities is essential for anticipating, preventing, and managing them effectively. Several key factors—including the pharmacologic targets of the drugs, host metabolism, genetic variability, and drug-drug interactions—contribute to the overall toxicity profile of oral agents.
Off-Target Toxicities in Cytotoxic Chemotherapy
Traditional oral chemotherapeutic agents, such as capecitabine and temozolomide, are inherently non-selective, targeting all rapidly dividing cells regardless of their malignant or healthy status. This explains the off-target damage observed in tissues with high turnover rates, particularly the gastrointestinal mucosa, bone marrow, and hair follicles. For instance, mucositis, diarrhea, and cytopenias frequently result from direct cytotoxic injury to epithelial stem cells and hematopoietic progenitors. These effects are dose-dependent and can become cumulative over multiple cycles of therapy.
- On-Target, Off-Tumor Effects in Targeted Therapies: In contrast to cytotoxic agents, targeted therapies are designed to selectively inhibit molecular pathways involved in tumor growth. However, these targets are often also expressed in normal tissues, leading to on-target, off-tumor effects. For example, epidermal growth factor receptor (EGFR) inhibitors such as erlotinib and osimertinib disrupt epithelial homeostasis in the skin and gastrointestinal tract, resulting in acneiform rash, xerosis, and diarrhea. Similarly, VEGF inhibitors may impair normal angiogenesis, causing hypertension and wound-healing complications. These toxicities are mechanistically predictable based on the expression patterns of the molecular targets in non-malignant tissues.
- Genetic and Metabolic Determinants of Toxicity: Individual variability in drug metabolism significantly impacts the severity of toxicity. One well-characterized example is dihydropyrimidine dehydrogenase (DPD) deficiency, which impairs the breakdown of fluoropyrimidines like capecitabine. Patients with DPYD gene mutations are at risk of life-threatening toxicity even at standard doses, including severe neutropenia, mucositis, and gastrointestinal bleeding. Routine testing for DPYD variants is now endorsed in several guidelines to reduce adverse outcomes. Similarly, polymorphisms in genes such as UGT1A1 (irinotecan metabolism) and CYP3A4/CYP3A5 (TKI metabolism) can alter plasma drug concentrations, leading to increased toxicity or therapeutic failure. These pharmacogenetic insights are increasingly being integrated into personalized treatment planning.
- Drug-Drug Interactions and Polypharmacy: Many oral chemotherapy agents are substrates, inhibitors, or inducers of cytochrome P450 enzymes, particularly CYP3A4, which plays a central role in hepatic drug metabolism. Concomitant administration of CYP3A4 inhibitors (e.g., azole antifungals, macrolide antibiotics) or inducers (e.g., rifampin, St. John’s Wort) can significantly alter drug exposure, leading to unexpected toxicity or reduced efficacy. This is particularly relevant in elderly patients or those with multiple comorbidities, where polypharmacy is common. In addition, interactions with acid-suppressing agents (e.g., PPIs) can impair absorption of pH-sensitive oral agents like erlotinib, further complicating therapeutic management.
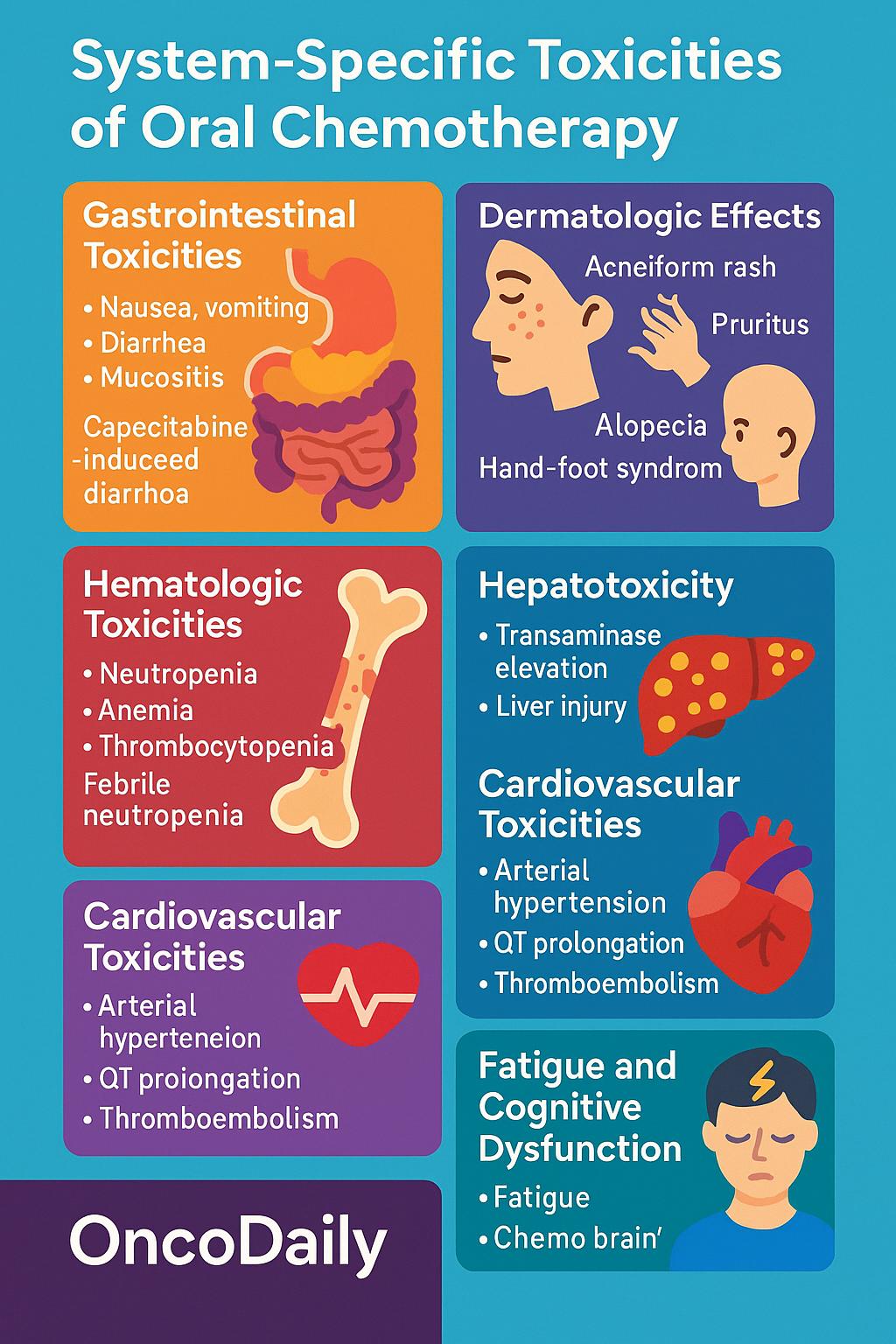
System-Specific Toxicities of Oral Chemotherapy Agents
Oral chemotherapy, while offering convenience and patient autonomy, is associated with a broad spectrum of adverse effects that vary in frequency, severity, and underlying mechanisms. Understanding the system-specific toxicities is essential for effective management and for tailoring therapy to individual patient needs. Below is an overview of the most common toxicities classified by organ system.
Gastrointestinal Toxicities
Gastrointestinal (GI) adverse events are among the most frequent and burdensome toxicities associated with oral chemotherapy. Agents such as capecitabine, temozolomide, and various tyrosine kinase inhibitors frequently induce nausea, vomiting, diarrhea, and mucositis, all of which can significantly impair nutritional status, hydration, and quality of life.
One hallmark example is capecitabine-induced diarrhea, which results from its conversion to 5-fluorouracil (5-FU) in the gastrointestinal mucosa via thymidine phosphorylase—an enzyme that is upregulated in tumor and normal epithelial tissues. The local accumulation of 5-FU leads to mucosal inflammation and epithelial cell death. Additionally, capecitabine is well known to cause hand-foot syndrome (palmar-plantar erythrodysesthesia), which, although classified dermatologically, shares mechanistic overlap with GI toxicity due to the drug’s activation in high perfusion areas.
Management strategies include prompt dose interruption or reduction, aggressive hydration, use of loperamide for diarrhea, and prophylactic antiemetics to control nausea. In severe or prolonged cases, hospitalization and parenteral support may be necessary to prevent electrolyte imbalances and renal dysfunction.
Dermatologic Effects
Cutaneous toxicities are common with oral targeted therapies, particularly EGFR and VEGF pathway inhibitors. Patients may experience acneiform rash, xerosis (dry skin), pruritus, paronychia, hand-foot syndrome, and alopecia, with varying degrees of severity.
The underlying mechanism is often on-target, off-tumor inhibition of EGFR signaling in keratinocytes, which disrupts skin barrier function and epidermal homeostasis. In VEGF inhibition, impaired angiogenesis may contribute to delayed wound healing and skin fragility. The rash typically develops within the first two weeks of therapy and is often localized to the face, scalp, and upper trunk.
Though not usually life-threatening, these skin toxicities can be distressing and lead to dose interruptions or discontinuation. Preventive strategies include the use of urea-based emollients, topical corticosteroids, oral tetracyclines such as doxycycline, and sun protection. Early dermatologic consultation improves outcomes and adherence.
Hematologic Toxicities
Hematologic side effects are most pronounced with cytotoxic oral agents such as temozolomide, etoposide, and cyclophosphamide, which target rapidly dividing hematopoietic precursors in the bone marrow. The most common manifestations include neutropenia, anemia, and thrombocytopenia, with potential for cumulative bone marrow suppression over successive treatment cycles.
Of particular concern is febrile neutropenia, which carries a risk of serious infection and requires immediate intervention. In patients receiving temozolomide for glioblastoma, prolonged lymphopenia is not uncommon and may predispose to opportunistic infections such as Pneumocystis jirovecii pneumonia (PJP).
Management strategies depend on severity, with options including dose reduction, treatment delay, or administration of granulocyte colony-stimulating factors (G-CSF). Regular monitoring of complete blood counts (CBC) is essential, particularly in the first two cycles of therapy when nadirs are most unpredictable.
Hepatotoxicity
Several oral agents, especially tyrosine kinase inhibitors (TKIs) such as pazopanib, lapatinib, and sunitinib, are associated with hepatotoxicity, which can range from mild transaminase elevation to fulminant hepatic failure. The incidence varies by agent and dose, but hepatotoxicity remains a leading cause of treatment discontinuation in oral targeted therapies.
Mechanistically, liver injury may result from mitochondrial dysfunction, oxidative stress, or idiosyncratic hypersensitivity reactions. Pazopanib, in particular, has been associated with cholestatic injury and bile duct damage, which can mimic autoimmune or obstructive liver disease.
Baseline and periodic liver function tests (LFTs) are recommended, with closer surveillance in patients with preexisting hepatic impairment. In cases of significant transaminase elevation (≥3× ULN with symptoms or ≥5× ULN without symptoms), therapy should be held and reassessed, with consideration of permanent discontinuation if abnormalities persist.
Cardiovascular Toxicities
Cardiovascular adverse effects are an important consideration, particularly in patients with pre-existing cardiac risk factors. VEGF inhibitors, including regorafenib and lenvatinib, are associated with arterial hypertension, which may be early-onset and dose-dependent. QT prolongation, seen with agents such as vandetanib and lapatinib, poses a risk for ventricular arrhythmias, particularly in the context of electrolyte imbalances.
Moreover, thromboembolic events, including deep vein thrombosis and pulmonary embolism, have been reported with both cytotoxic and targeted oral therapies, possibly related to endothelial injury and altered coagulation pathways.
To mitigate these risks, patients should undergo baseline and periodic ECGs, electrolyte monitoring, and blood pressure assessments. Antihypertensive therapy may be initiated preemptively, and dose modification may be required if cardiovascular events occur.
Fatigue and Cognitive Dysfunction
Cancer-related fatigue is a frequently reported but often underappreciated side effect of oral chemotherapy. Unlike other toxicities, it lacks objective biomarkers and can be multifactorial in origin. Contributing factors include anemia, systemic inflammation, cytokine dysregulation, sleep disturbances, and cumulative psychological stress.
In parallel, some patients experience subjective cognitive decline, often described as “chemo brain” or “chemo fog,” characterized by reduced attention, memory lapses, and slowed mental processing. These effects may persist beyond the treatment period and impair daily functioning and quality of life.
Management is largely supportive, emphasizing restorative sleep, physical activity, nutritional support, and psychological counseling. Screening tools such as the Brief Fatigue Inventory (BFI) can help quantify symptom burden and guide interventions.
Populations at Increased Risk for Chemotherapy-Related Toxicities
Certain patient populations demonstrate heightened vulnerability to the adverse effects of oral chemotherapy due to physiological, genetic, and behavioral factors that alter drug metabolism, clearance, or response. Older adults, in particular, represent a growing demographic in oncology and are frequently affected by polypharmacy, altered pharmacokinetics, and age-related organ dysfunction. Age-associated declines in renal and hepatic function, coupled with changes in body composition—such as increased fat-to-lean mass ratio—can modify drug distribution and clearance. In addition, the presence of multiple comorbidities increases the likelihood of drug-drug interactions and limits the patient’s physiologic reserve, rendering them more susceptible to cumulative toxicity, fatigue, and myelosuppression.
Renal and hepatic impairment significantly affect the metabolism and elimination of many oral chemotherapeutic agents. Drugs such as capecitabine, methotrexate, and various tyrosine kinase inhibitors require intact renal or hepatic pathways for clearance. Impaired function can lead to drug accumulation, prolonged exposure, and elevated toxicity, particularly mucositis, diarrhea, or hematologic suppression. Standard dosing may be unsafe in these patients, necessitating individualized dose adjustments based on creatinine clearance or liver function tests.
Genetic polymorphisms in drug-metabolizing enzymes further complicate toxicity profiles. A well-established example is deficiency in dihydropyrimidine dehydrogenase (DPD), caused by mutations in the DPYD gene. Patients with partial or complete DPD deficiency are at high risk for severe, sometimes fatal toxicity when treated with fluoropyrimidines such as capecitabine. Manifestations include profound neutropenia, mucositis, diarrhea, and neurotoxicity. As a result, many international guidelines now recommend pharmacogenomic screening for DPYD variants prior to initiating therapy, enabling risk stratification and dose individualization.
In addition, patients who concurrently use complementary and alternative medicine (CAM)—including herbal supplements, traditional remedies, or over-the-counter botanicals—may unknowingly alter the pharmacokinetics of oral chemotherapy. Several commonly used herbs, such as St. John’s Wort, ginseng, and turmeric, can induce or inhibit cytochrome P450 enzymes, particularly CYP3A4, leading to unpredictable changes in systemic drug levels. These interactions may either exacerbate toxicity or reduce therapeutic efficacy. Given the high prevalence of unreported CAM use in oncology patients, clinicians must proactively inquire about non-prescription products and consider potential interactions during treatment planning.
Current Strategies for Toxicity Management
Effective management of oral chemotherapy toxicity relies on a combination of evidence-based clinical guidelines, proactive symptom control, and multidisciplinary coordination. Central to this approach are dose modification protocolsguided by the Common Terminology Criteria for Adverse Events (CTCAE), which provide standardized thresholds for adjusting or withholding treatment based on toxicity grade. These protocols help clinicians balance efficacy with safety, reducing the risk of severe complications while maintaining therapeutic intent.
Supportive care is integral to minimizing patient discomfort and preventing treatment discontinuation. Antiemetic regimens—including 5-HT₃ antagonists and NK1 receptor blockers—are used prophylactically for agents with known emetogenic potential. Antidiarrheals such as loperamide or octreotide are employed early in treatment to mitigate gastrointestinal side effects, especially in patients receiving capecitabine or TKIs. Dermatologic care, including the use of urea-based emollients, corticosteroids, and tetracyclines, has shown efficacy in managing cutaneous toxicities from EGFR inhibitors. In cases of chemotherapy-induced neutropenia, granulocyte colony-stimulating factors (G-CSFs) are recommended to prevent febrile episodes and allow continuation of therapy without dose compromise.
Emerging models of care increasingly incorporate telemonitoring and digital health tools to facilitate real-time toxicity detection. Electronic patient-reported outcome (ePRO) systems, mobile applications, and automated symptom alerts enable earlier intervention, reduce emergency visits, and improve quality of life. Studies have shown that integrating ePRO platforms into routine oncology practice leads to improved survival outcomes and better adherence to therapy.
A multidisciplinary approach is essential for comprehensive toxicity management. Oncology nurses monitor for adverse effects and educate patients on self-care strategies. Clinical pharmacists evaluate drug interactions and adjust regimens for organ dysfunction or pharmacogenetic risk. Nutritionists provide guidance on managing anorexia, mucositis, or diarrhea-related malnutrition. This collaborative model ensures timely identification and resolution of toxicities, ultimately improving treatment continuity.
Emerging Approaches and Future Directions
The future of oral chemotherapy toxicity management lies in precision medicine, where treatment is tailored not only to the cancer’s molecular profile but also to the patient’s unique pharmacogenomic and physiological characteristics. Pharmacogenomic testing is increasingly used to guide personalized dosing, particularly for agents like capecitabine, where variants in the DPYD gene significantly affect drug metabolism and toxicity risk. Incorporating such testing into routine oncology practice can help identify high-risk patients before treatment begins, reducing the incidence of severe adverse events.
At the forefront of innovation are AI-driven toxicity prediction models that leverage large-scale real-world data, including electronic health records and patient-reported outcomes. These tools aim to identify patterns and early warning signs of adverse effects, allowing clinicians to intervene proactively. Machine learning algorithms are also being developed to optimize dose selection and scheduling based on individual risk profiles, comorbidities, and treatment history.
Additionally, novel drug formulations and delivery systems are under active investigation to improve tolerability. Nanoparticle-based carriers, liposomal delivery, and prodrug designs are being developed to enhance tumor targeting, reduce systemic exposure, and minimize off-target toxicity. These technologies offer the potential to expand the therapeutic window and allow for more aggressive dosing in patients who would otherwise be ineligible.
Despite these advances, there remains a critical need for robust post-marketing surveillance to capture real-world toxicity data beyond the controlled settings of clinical trials. Integration of patient-reported outcomes (PROs) into both research and clinical care can provide invaluable insights into the subjective burden of treatment and inform more responsive, patient-centered management strategies. Together, these evolving approaches promise to redefine how we anticipate, prevent, and manage the side effects of oral chemotherapy.
You Can Watch More on OncoDaily Youtube TV
Written by Toma Oganezova, MD
FAQ
What are common side effects of oral chemotherapy?
Fatigue, nausea, diarrhea, rash, liver issues, and blood count changes are common.
Are side effects different from IV chemotherapy?
Yes, oral agents often have different toxicity profiles and require different management strategies.
Can oral chemotherapy cause liver or heart problems?
Yes, certain drugs can cause hepatotoxicity or cardiovascular issues like hypertension or QT prolongation.
What should I avoid while on oral chemo?
Avoid grapefruit juice, certain supplements, and medications that interact with CYP3A4 unless approved by your doctor.
Is fatigue from oral chemo treatable?
Supportive care, sleep hygiene, nutrition, and exercise may help reduce fatigue. Always consult your care team.
Is hair loss common with oral chemotherapy?
It depends on the drug. Some, like capecitabine, may cause hair thinning, but not all do.
Can diet affect oral chemotherapy side effects?
Yes. Nutritional support can help manage mucositis, diarrhea, and overall energy.
Are drug interactions common with oral chemotherapy?
Yes. Many oral agents interact with other medications, especially those affecting the liver (CYP enzymes).
Can I take herbal supplements with oral chemo?
It’s risky. Always consult your oncologist, as some herbs interfere with drug metabolism.
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
