Selpercatinib (Retevmo): Uses in Cancer, Side Effects, Dosage, Expectations, and More
Selpercatinib is a targeted therapy for cancers driven by RET gene alterations, including NSCLC and thyroid cancers. It blocks abnormal RET proteins that fuel tumor growth. On May 8, 2020, the FDA granted accelerated approval for adults and pediatric patients (12+) with metastatic RET fusion-positive NSCLC, advanced or metastatic RET-mutant medullary thyroid cancer (MTC), and radioactive iodine-refractory RET fusion-positive thyroid cancer.
On September 21, 2022, the FDA granted accelerated approval for adult patients with locally advanced or metastatic solid tumors harboring RET gene fusions that progressed after prior treatment or had no satisfactory alternatives.
Which Company produced Retevmo?
Retevmo is produced by Eli Lilly and Company, a global pharmaceutical company headquartered in Indianapolis, Indiana, USA. Founded in 1876, Eli Lilly is known for its innovations in oncology, diabetes, immunology, and neuroscience. The company has developed several breakthrough drugs, including insulin, monoclonal antibodies, and targeted cancer therapies.
Innovent Biologics announced that China’s NMPA has approved selpercatinib (40mg & 80mg capsules) for adults and patients 12+ with RET fusion-positive NSCLC, medullary thyroid cancer (MTC), or other advanced thyroid cancers requiring systemic therapy and refractory to radioactive iodine.
Originally developed by Eli Lilly, selpercatinib is a selective RET kinase inhibitor. Through a strategic partnership, Innovent holds exclusive commercialization rights in China and will manage its pricing, import, marketing, and distribution, leveraging its strong oncology network to expand patient access.
How does Selpercatinib work?
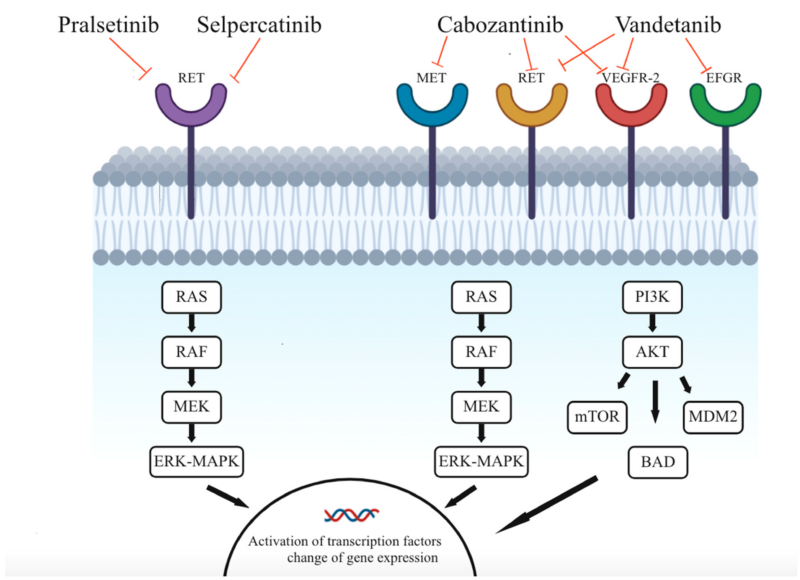
In normal cells, the RET gene plays a role in cell growth and development. However, in some cancers like NSCLC and thyroid cancer, mutations or fusions in RET cause it to become overactive, sending constant signals that drive uncontrolled tumor growth.
Selpercatinib works by blocking this abnormal RET activity. It binds to the RET protein’s ATP-binding site, preventing it from sending growth signals. Without these signals, cancer cells stop multiplying and eventually die. Unlike older drugs, selpercatinib is highly selective for RET, making it more effective while reducing side effects. It also crosses the blood-brain barrier, helping to treat brain metastases in patients with advanced disease.
Milling, R.V.; Grimm, D.; Krüger, M.; Grosse, J.; Kopp, S.; Bauer, J.; Infanger, M.; Wehland, M. Pazopanib, Cabozantinib, and Vandetanib in the Treatment of Progressive Medullary Thyroid Cancer with a Special Focus on the Adverse Effects on Hypertension. Int. J. Mol. Sci. 2018, 19, 3258.
What Cancers Is Selpercatinib Approved to Treat?
Selpercatinib is a targeted therapy for cancers driven by RET gene alterations, including NSCLC and thyroid cancers. It blocks abnormal RET proteins to slow tumor growth.
🔹 May 8, 2020 – First FDA Approval (Accelerated)
The FDA granted accelerated approval for selpercatinib to treat:
- RET fusion-positive non-small cell lung cancer (NSCLC)
- RET-mutant medullary thyroid cancer (MTC)
- RET fusion-positive thyroid cancer
This initial approval was based on early results from the LIBRETTO-001 clinical trial, where many patients experienced strong tumor responses—even after other treatments had failed.
🔹 September 21, 2022 – Tumor-Agnostic Approval (Accelerated)
Selpercatinib became the first RET inhibitor approved for adults with advanced solid tumors that have RET gene fusions, no matter where the cancer started in the body. This “tumor-agnostic” approach is part of precision oncology, where treatments are based on the tumor’s genetic profile—not just its location.
🔹 May 29, 2024 – Pediatric Approval (Accelerated)
The FDA extended selpercatinib’s use to children aged 2 years and older with:
- RET-altered thyroid cancer that has spread (metastatic)
- RET-altered solid tumors
This approval gives younger patients access to personalized treatment options for rare cancers driven by RET mutations.
🔹 June 12, 2024 – Full FDA Approval for RET Fusion-Positive Thyroid Cancer
Based on longer-term data, the FDA granted traditional approval for selpercatinib in RET fusion-positive thyroid cancer, confirming its effectiveness and safety over time.
🔹 September 27, 2024 – Full FDA Approval for RET-Mutant Medullary Thyroid Cancer
Selpercatinib received full approval for treating RET-mutant medullary thyroid cancer in adults and children aged 2 years and older, marking a key advancement for patients with this rare type of thyroid cancer.
What research is behind the approval?
The approvals of Retevmo were based on data from several key clinical trials that assessed its safety and efficacy in treating RET-driven cancers. The primary research behind its approvals includes:
LIBRETTO-001 Trial
This was a pivotal, multicenter, open-label trial that enrolled patients with RET fusion-positive non-small cell lung cancer (NSCLC), RET-mutant medullary thyroid cancer (MTC), and RET fusion-positive thyroid cancer. The results were published in NEJM on August 26, 2020.
RET fusions are found in 1-2% of NSCLC cases, and this trial focused on its efficacy in chemotherapy-pretreated and untreated patients. In 105 previously treated patients, the objective response rate (ORR) was 64%, with a median response duration of 17.5 months. In 39 untreated patients, the ORR was 85%, and 90% of responses were ongoing at 6 months. The drug also showed a 91% intracranial response in those with CNS metastases. Common grade 3+ side effects included hypertension, elevated liver enzymes, and hyponatremia. Overall, Selpercatinib demonstrated durable efficacy and low-grade toxicities in both groups.

Selpercatinib was tested in patients with RET-mutant medullary thyroid cancer (MTC) and RET fusion-positive thyroid cancer. The response rate for patients with treated MTC was 69%, with a 1-year progression-free survival (PFS) of 82%. In untreated MTC patients, the response rate was 73%, and 1-year PFS was 92%. For RET fusion-positive thyroid cancer, the response rate was 79%, with 1-year PFS of 64%. The most common grade 3+ side effects were hypertension and liver enzyme increases. Overall, Retevmo showed durable efficacy and low-grade toxicities in these cancers.
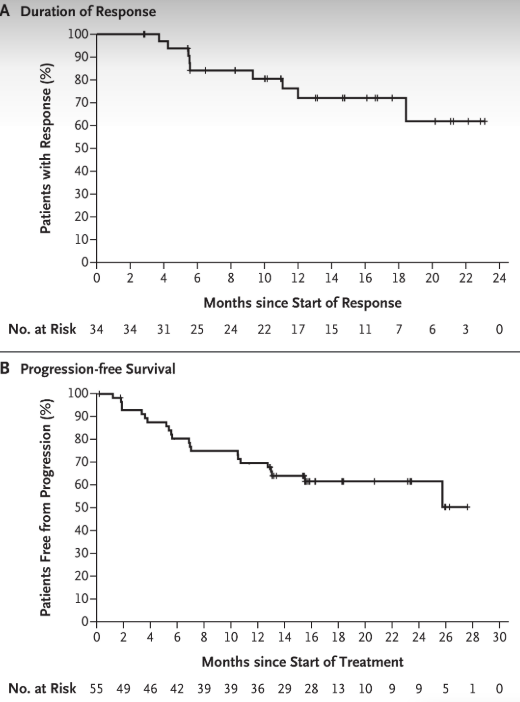
Subgroup Analysis of Pediatric Patients
The approval of selpercatinib (Retevmo) for children as young as 2 years old with RET-altered thyroid cancer or solid tumors was supported by the LIBRETTO-121 trial (NCT03899792). This ongoing study is the first to test selpercatinib in pediatric and adolescent patients with RET-driven cancers.
As of early 2023, 27 patients aged 2–20 were treated, including those with medullary and papillary thyroid cancers. The overall response rate was 83.3%, with 100% response in papillary thyroid cancer and 83% in medullary thyroid cancer. Most children remained on treatment, and side effects were manageable. Importantly, no one stopped treatment due to side effects.
The results of LIBRETTO-121 were published in JCO (Journal of Clinical Oncology) in May 2024, reinforcing selpercatinib’s effectiveness and safety for younger patients.
Selpercatinib vs Standard Therapies in RET-Mutant Medullary Thyroid Cancer
Published in The New England Journal of Medicine (NEJM) in 2023, the LIBRETTO-531 phase 3 trial compared selpercatinib to standard treatments (cabozantinib or vandetanib) in patients with advanced RET-mutant medullary thyroid cancer.
Selpercatinib significantly improved progression-free survival and treatment failure–free survival, with a 69.4% response rate vs. 38.8% in the control group. Fewer patients needed to stop treatment due to side effects.
These results confirmed selpercatinib’s superior efficacy and supported its FDA approval for this cancer type.
Selpercatinib Combinations and Treatment Outcomes
A study published in Clinical Cancer Research on January 4, 2021, explored the combination of selpercatinib and crizotinib to overcome MET-dependent resistance in RET fusion–positive NSCLC. Researchers identified MET amplification as a mechanism of acquired resistance in patients treated with selpercatinib. In post-treatment biopsies, four patients showed MET amplification, with one case displaying it before Retevmo therapy.
Laboratory findings confirmed that increased MET expression led to resistance, which could be countered by combining selpercatinib with crizotinib, a MET inhibitor. Single-patient protocols demonstrated clinical activity, with one response lasting 10 months. These results highlight MET dependence as a recurrent and targetable resistance mechanism in RET fusion–positive NSCLC.
A PubMed case report (2023) describes a patient with ISOC1-RET fusion NSCLC who initially responded to selpercatinib but developed resistance due to MET amplification. Combining selpercatinib with capmatinib restored a complete response, though pneumonitis emerged as a side effect. This highlights the potential of dual RET and MET inhibition to overcome resistance while underscoring the need for further safety evaluations.
In a PubMed 2024 case report, a 68-year-old man with EGFR L858R-positive lung adenocarcinoma developed resistance to dacomitinib due to a CCDC6-RET fusion. After partial response to chemotherapy, disease progression led to hydrocephalus. The combination of dacomitinib and selpercatinib improved his symptoms. This case underscores the role of NGS in detecting RET fusions and suggests dacomitinib plus selpercatinib as a potential treatment option.
Read about Immunotherapy for Lung Cancer on OncoDaily.
Selpercatinib side effects and its management
Retevmo can cause side effects, but they are usually manageable. The most common are hypertension, elevated liver enzymes, fatigue, and diarrhea. Regular blood pressure monitoring and antihypertensive treatment can help manage hypertension.
Liver function should be monitored, and if enzyme levels rise significantly, dose adjustments may be needed. Fatigue can be managed with rest and by addressing underlying causes like anemia, while diarrhea can be controlled with hydration and antidiarrheal medications.
Proteinuria and electrolyte imbalances, such as hyponatremia, should also be monitored. If significant, dose adjustments or treatment interruptions may be required. Lymphopenia, which lowers immunity, can be monitored with blood tests. Neurological symptoms, such as headaches, should be watched in patients with CNS metastases. Skin reactions, like rashes, can be managed with topical treatments.
Serious side effects, like severe liver toxicity or allergic reactions, are rare but may require discontinuation of the drug. Regular monitoring and timely interventions help manage these effects, allowing patients to benefit from Selpercatinib’s effectiveness.

What is the Recommended Dosage of Selpercatinib?
The recommended dosage of Retevmo depends on the patient’s weight and the type of cancer being treated. For patients with metastatic RET fusion-positive non-small cell lung cancer (NSCLC), those weighing less than 50 kg should take 120 mg twice daily, while patients weighing 50 kg or more should take 160 mg twice daily.
The same dosage applies for patients with advanced or metastatic RET-mutant medullary thyroid cancer (MTC) requiring systemic therapy. Treatment should continue until disease progression or the occurrence of unacceptable side effects.
How is Selpercatinib administered?
Retevmo is taken orally, with or without food, unless taken with a proton pump inhibitor (PPI), in which case it should be taken with food. Capsules or tablets should be swallowed whole and not crushed or chewed. Capsules should not be given to children who cannot swallow them.
If a dose is missed and it’s more than 6 hours until the next dose, take it as soon as possible. If you vomit after taking a dose, do not take an extra dose; just resume the next scheduled dose.
Store at 20-25ºC, with allowed temperature fluctuations between 15-30ºC.

Learn more about Thyroid Cancer on OncoDaily.
What to Avoid During Selpercatinib Treatment?
During treatment with selpercatinib, patients should avoid certain substances and activities to ensure the medication works effectively and reduce potential risks.
Grapefruit and grapefruit juice should be avoided, as they can increase the levels of Retevmo in the body, potentially leading to serious heart rhythm issues. Additionally, combining selpercatinib with strong CYP3A inhibitors, such as certain antifungals, antibiotics, or HIV protease inhibitors, can elevate selpercatinib concentrations, increasing the risk of adverse effects. If coadministration is necessary, dose adjustments may be required.
Medications that prolong the QTc interval should also be avoided, as selpercatinib may have additive effects, heightening the risk of arrhythmias. Patients should consult their healthcare provider before starting any new medications, including over-the-counter drugs or herbal supplements, to prevent potential interactions.
Since selpercatinib can cause hypertension, regular monitoring of blood pressure is essential. Blood pressure should be controlled before starting treatment, and it should be checked regularly during therapy.
Selpercatinib effectiveness over time
The final safety and efficacy results of Retevmo in RET fusion–positive non–small cell lung cancer (NSCLC) were published in the Journal of Clinical Oncology on February 21, 2025, in the study titled Selpercatinib in RET Fusion–Positive Non–Small Cell Lung Cancer: Final Safety and Efficacy, Including Overall Survival, From the LIBRETTO-001 Phase I/II Trial.
The LIBRETTO-001 trial demonstrated that Retevmo provides durable responses in patients with RET fusion–positive NSCLC. Treatment-naive patients achieved an objective response rate (ORR) of 84%, with a median duration of response (DoR) of 20.2 months. In previously treated patients, the ORR was 61%, with a median DoR of 28.6 months. The drug also showed significant intracranial activity, with an 85% ORR among patients with measurable CNS metastases.
Long-term follow-up data confirmed the sustained efficacy of selpercatinib, with a median progression-free survival (PFS) of 22.0 months in treatment-naive patients and 24.9 months in those previously treated. Additionally, the 3-year overall survival rates were 66% for treatment-naive patients and 57% for pretreated patients, highlighting the long-term benefits of selpercatinib.
Ongoing trials with Selpercatinib
A Phase II clinical trial (NCT06458036) is investigating the combination of selpercatinib followed by radioactive iodine (RAI) therapy in children, adolescents, and young adults newly diagnosed with differentiated thyroid cancers harboring RET fusions. The study aims to evaluate the pulmonary structural response rate after 18 months of treatment. This open-label, non-randomized trial is exempt from Investigational New Drug requirements per the FDA.
FAQ
What is Selpercatinib used for?
Selpercatinib is a targeted therapy approved for treating certain cancers with RET gene alterations, including non-small cell lung cancer (NSCLC), medullary thyroid cancer (MTC), and other thyroid cancers that are advanced or metastatic.
How does Selpercatinib work?
Selpercatinib is a RET kinase inhibitor that blocks abnormal RET signaling in cancer cells, preventing tumor growth and spread.
How is Selpercatinib administered?
Selpercatinib is taken orally as a capsule, usually twice daily, with or without food. The dosage is based on body weight and specific cancer type.
What are the common side effects of Selpercatinib?
Common side effects include hypertension, dry mouth, diarrhea, liver enzyme elevation, fatigue, and swelling. More serious side effects may include QT prolongation and liver toxicity.
Does Selpercatinib interact with other medications?
Yes, Selpercatinib can interact with certain drugs, including strong CYP3A inhibitors and inducers, which may affect its effectiveness or cause side effects.
What should patients expect during Selpercatinib treatment?
Patients should expect regular monitoring of blood pressure, liver function, and heart rhythm.
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023