
What Is Heavy Ion Radiotherapy and How It Works? Pros and Cons
Heavy ion radiotherapy (HIR) is an advanced cancer treatment using accelerated heavy ions to precisely destroy tumors, especially deep-seated and radiation-resistant ones. Unlike X-ray therapy, HIR delivers a concentrated radiation dose at the tumor’s depth, minimizing damage to healthy tissue. It’s effective against resistant tumors and offers shorter treatment times. However, it requires high initial costs and specialized technology, limiting accessibility.
What Is Heavy Ion Radiotherapy?
Heavy ion radiotherapy (HIR) is a sophisticated form of cancer treatment that employs accelerated heavy ions, most commonly carbon, to precisely target and destroy tumors. This method distinguishes itself from traditional radiotherapy by leveraging the unique physical and biological properties of these heavy ions.
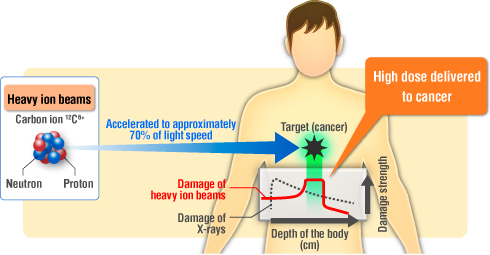
In HIR, carbon or other heavy ions are accelerated to a substantial fraction of the speed of light. These high-energy ions are then directed towards the tumor, delivering a concentrated dose of radiation directly to the cancerous cells. Unlike conventional X-ray therapy, which deposits energy along its entire path through the body, heavy ion therapy exhibits a distinct energy deposition pattern. The radiation dose increases with depth, reaching a maximum at a specific point known as the Bragg peak, which can be precisely positioned within the tumor. This allows for a highly targeted delivery of radiation, minimizing damage to surrounding healthy tissues.
Furthermore, heavy ions possess a higher relative biological effectiveness (RBE) compared to X-rays. This means they are more effective at damaging cancer cell DNA, particularly the complex double-strand breaks that are difficult for cells to repair. This increased biological effectiveness is especially beneficial for treating tumors that are resistant to conventional radiation, such as hypoxic tumors or those with complex DNA repair mechanisms. The combination of precise physical targeting and enhanced biological effectiveness makes heavy ion radiotherapy a powerful tool in the fight against cancer.
How Does Heavy Ion Radiotherapy Work?
Heavy ion radiotherapy (HIR) operates through a sophisticated process that begins with the acceleration of heavy ions, typically carbon, to a substantial fraction of the speed of light. These high-energy ions are then magnetically guided and precisely directed towards the tumor site within the patient’s body. The delivery system is designed to ensure that the ion beam’s path and energy are meticulously controlled.
A key aspect of HIR’s effectiveness lies in the Bragg peak phenomenon. As the heavy ions travel through tissue, they deposit relatively little energy until they reach a specific depth, at which point they release a concentrated burst of energy. This peak of energy deposition, the Bragg peak, can be precisely positioned within the tumor, allowing for a high dose of radiation to be delivered directly to the cancerous cells.
This unique energy deposition pattern is what allows HIR to minimize damage to surrounding healthy tissue. Unlike conventional X-ray therapy, which deposits energy along its entire path through the body, HIR delivers the majority of its radiation dose at the tumor’s location. By carefully controlling the energy and trajectory of the ion beam, clinicians can ensure that the Bragg peak aligns with the tumor’s shape and depth, effectively targeting cancer cells while sparing the healthy tissues located before and beyond the tumor. This precision is further enhanced by advanced imaging and treatment planning, allowing for highly individualized treatment strategies.
source: www. particle.or.jp
What Are the Main Types of Heavy Ion Radiotherapy?
Heavy ion radiotherapy (HIR) primarily manifests as a form of external beam radiation therapy (EBRT), where accelerated heavy ions are directed from an external source towards the tumor. This method stands apart from conventional X-ray and proton therapies due to the unique physical properties of the heavy ions used.
Within EBRT, HIR distinguishes itself through its ability to deliver a highly concentrated dose of radiation directly to the tumor, minimizing damage to surrounding healthy tissues. This is achieved through the Bragg peak phenomenon, where the ions deposit the majority of their energy at a specific depth within the tissue, precisely targeting the tumor. Advanced techniques within HIR include the use of rotating gantries and scanning systems, enabling the delivery of heavy ion beams from multiple angles, thus enhancing the precision and adaptability of the treatment.
When compared to proton therapy, HIR, particularly with carbon ions, offers a higher relative biological effectiveness (RBE). This means that heavy ions are more effective at damaging cancer cell DNA, especially in tumors resistant to conventional radiation. While proton therapy also utilizes the Bragg peak, it exhibits greater lateral scattering and a lower RBE than heavy ion therapy.
Conventional X-ray radiation therapy, in contrast, deposits energy along its entire path through the body, affecting both healthy tissues and the tumor. It lacks the precise targeting capabilities of both proton and heavy ion therapies. Heavy ion therapy also allows for hypofractionation, which means fewer treatment sessions are needed, something that can be more difficult to achieve with X-ray radiotherapy. Thus, heavy ion radiotherapy represents a more advanced and targeted approach to cancer treatment, offering significant advantages over traditional radiation methods.

Read OncoDaily’s Special Article About Proton Therapy
What Types of Cancer Are Treated with Heavy Ion Radiotherapy?
Heavy ion radiotherapy (HIR) has demonstrated effectiveness in treating a diverse range of cancers, particularly those that are challenging to address with conventional radiation therapies. This method has shown promise in treating pancreatic cancer, where its precision targeting and high biological effectiveness can be crucial in managing tumors located near critical organs. Sarcomas, including osteosarcoma, which often exhibit resistance to X-ray radiation, have also shown positive responses to HIR due to its ability to deliver a concentrated dose of radiation directly to the tumor.
Head and neck cancers, where preserving healthy tissue is paramount, benefit from HIR’s precise targeting capabilities, minimizing side effects. Recurrent tumors, which may have developed resistance to previous radiation treatments, can also be effectively addressed with HIR’s high relative biological effectiveness. Lung cancer and liver cancer, particularly those inoperable or located near sensitive structures, can be treated with HIR’s ability to precisely deliver high doses of radiation.
Byun et al., in Clinical and Molecular Hepatology, highlight carbon ion radiotherapy (CIRT) as a promising treatment for hepatocellular carcinoma (HCC). CIRT’s unique physical and biological properties, including the Bragg Peak and high relative biological effectiveness, enable precise tumor targeting and destruction, even in radioresistant and hypoxic areas. Clinical outcomes show excellent local control with minimal side effects. CIRT is a viable option for complex HCC cases, including those with poor liver function, large tumors, and tumors near critical organs. Further studies are needed to define definitive indications and compare CIRT with other treatments.
Heavy Ion Radiotherapy Side Effects: What Should You Expect During Heavy Iron Radiotherapy?
Heavy ion radiotherapy (HIR) is designed to minimize side effects compared to conventional radiation therapy, primarily due to its precise targeting capabilities. By concentrating the radiation dose within the tumor while sparing surrounding healthy tissues, HIR reduces the likelihood of widespread tissue damage. However, like any radiation therapy, HIR can still result in both short-term and long-term side effects, which vary depending on the treatment site and individual patient factors.
Short-Term Side Effects of Heavy Ion Radiotherapy
Short-term side effects of heavy ion radiotherapy are typically related to the immediate effects of radiation on the treated area. For instance, patients undergoing HIR for head and neck cancers might experience mucositis, or inflammation of the mucous membranes, leading to discomfort during swallowing. Skin reactions, such as redness or irritation, can also occur at the entry point of the ion beam. These short-term effects generally resolve after the completion of treatment.
Schulz-Ertner et al. (Radiotherapy and Oncology, 2002) reported on 37 skull base chordoma and chondrosarcoma patients treated with carbon ion therapy (60 GyE). Acute toxicities (≤°III) included skin reactions (n=4), mucositis (n=8), otitis/middle ear effusion (n=4), sinusitis (n=4), nausea/weight loss (n=1), temporal lobe edema (n=1), and worsened pre-existing neurological symptoms (n=2). The study concluded scanning beam delivery was safe with no unexpected acute dose-limiting toxicity, showing improved toxicity compared to passive beam shaping.
Long-Term Side Effects of Heavy Ion Radiotherapy
Long-term side effects are less common but can be more significant. They may include fibrosis, or the thickening and scarring of tissue, in the treated area. The risk of secondary cancers, though generally low, is also a concern, particularly in pediatric patients. The potential for damage to critical organs, such as the spinal cord or intestines, exists, but HIR’s precise targeting significantly reduces this risk. The severity and likelihood of long-term side effects are influenced by factors such as the total radiation dose, the specific ions used, and the patient’s overall health. While HIR is designed to minimize these effects, careful monitoring and follow-up are essential to manage any potential complications.
Eley et al. (2021) in Cancers explored heavy ion minibeam therapy for brain cancers, aiming to improve the therapeutic ratio. Juvenile rats were irradiated with 120 MeV lithium-7 ions (20 Gy) in either solid or minibeam configurations. After 6 months, both solid and minibeam irradiation resulted in cognitive impairment compared to controls. Results showed no apparent reduction in neurotoxicity with minibeams under the tested conditions. This study investigated late-term effects and potential toxicities of heavy ion minibeam therapy in normal brain tissue.
Managing Short-Term Side Effects of Heavy Ion Radiotherapy
Managing the short-term side effects of heavy ion radiotherapy involves a proactive approach, tailored to the specific area being treated and the individual patient’s response. Because heavy ion therapy delivers a concentrated dose of radiation, immediate effects are typically localized to the treatment site.
For instance, if the head and neck region is targeted, patients may experience mucositis, which can cause discomfort during eating and swallowing. In these cases, supportive care might include pain management, dietary adjustments to soft or liquid foods, and specialized mouthwashes to soothe inflamed tissues. Skin reactions, such as redness or irritation, can also occur at the beam’s entry point. These are often managed with topical creams and gentle skin care practices.
It’s crucial that patients communicate openly with their healthcare team about any discomfort they experience. This allows for prompt intervention and personalized management strategies. Regular monitoring during and immediately following treatment sessions enables the healthcare team to identify and address potential side effects early on. Supportive care, such as nutritional counseling and pain management, plays a significant role in mitigating these short-term effects, ensuring patients can maintain their quality of life throughout the treatment process.
Reducing the Risk of Long-Term Side Effects of Heavy Ion Radiotherapy
Minimizing the risk of long-term side effects following heavy ion therapy hinges on a combination of diligent monitoring, proactive intervention, and healthy lifestyle practices. Regular follow-up imaging, such as MRI and CT scans, plays a crucial role in detecting any subtle changes in the treated area. This allows for early identification of potential complications, such as fibrosis or the development of secondary cancers, enabling timely intervention.
Physical therapy is essential in preventing and managing fibrosis, a common long-term side effect. Targeted exercises and stretching regimens can help maintain flexibility and mobility in the treated area, reducing the risk of stiffness and pain. Early intervention with physical therapy can prevent the progression of fibrosis and improve functional outcomes.
Lifestyle modifications are equally important in promoting recovery and reducing the risk of long-term complications. A healthy diet, rich in fruits, vegetables, and lean proteins, provides the body with the nutrients it needs to repair and regenerate tissues. Regular exercise, tailored to individual capabilities, enhances cardiovascular health, improves circulation, and supports overall well-being. Stress management techniques, such as meditation, yoga, or deep breathing exercises, can help mitigate the psychological impact of cancer treatment and promote emotional resilience. By adopting these preventive measures, patients can significantly reduce the risk of long-term side effects and enhance their quality of life following heavy ion therapy.
Pros and Cons of Heavy Ion Radiotherapy
Heavy ion radiotherapy (HIR) presents a compelling set of advantages and drawbacks when considered as a cancer treatment modality. The primary advantage of HIR lies in its precision. The unique physical properties of heavy ions, particularly the Bragg peak phenomenon, allow for a highly concentrated radiation dose to be delivered directly to the tumor, minimizing damage to surrounding healthy tissues. This precision is particularly beneficial for tumors located near critical organs or in areas where surgical intervention would be challenging. Furthermore, heavy ions exhibit a higher relative biological effectiveness (RBE), meaning they are more effective at damaging cancer cell DNA, especially in tumors resistant to conventional radiation.
HIR also offers the potential for shorter treatment durations compared to traditional radiotherapy, which can enhance patient convenience and reduce overall treatment burden. The ability to treat radio-resistant tumors, such as certain sarcomas or hypoxic tumors, is another significant advantage. Additionally, emerging research suggests that HIR may stimulate the immune system, potentially contributing to long-term tumor control.
However, HIR also presents certain drawbacks. The initial capital cost of establishing heavy ion therapy centers is substantial, limiting its accessibility. The technology involved is complex, requiring specialized expertise and infrastructure. While HIR minimizes damage to healthy tissues, potential long-term side effects, such as fibrosis or secondary cancers, remain a concern.
How to Prepare for Heavy Ion Radiotherapy?
Preparing for heavy ion radiotherapy (HIR) involves a series of meticulously planned steps to ensure the treatment’s accuracy and effectiveness. The process begins with initial consultations where the patient’s medical history is thoroughly reviewed, and a comprehensive physical examination is conducted. This initial phase also includes detailed imaging scans, such as CT, MRI, and PET, to precisely locate and characterize the tumor. These scans are critical for developing an individualized treatment plan.
Following the initial consultations and imaging, a custom immobilization device is created to ensure the patient maintains a consistent and precise position during each treatment session. This device is tailored to the patient’s body shape and the specific treatment area, minimizing movement and ensuring accurate beam delivery. Simulation sessions are then conducted, where the patient is positioned using the immobilization device, and further imaging is performed to refine the treatment plan. This simulation allows the radiation therapy team to map out the exact angles and depths of the ion beams, ensuring optimal targeting of the tumor while sparing healthy tissues.
In addition to these technical preparations, patients are provided with lifestyle and dietary recommendations to support their overall well-being during treatment. Maintaining a healthy diet, rich in fruits, vegetables, and lean proteins, is essential for providing the body with the nutrients needed for tissue repair and recovery. Patients may also be advised to engage in gentle exercise, as tolerated, to maintain strength and flexibility. Stress management techniques, such as meditation or deep breathing exercises, can help patients cope with the emotional challenges of cancer treatment. Open communication with the healthcare team is paramount, allowing patients to address any concerns or questions they may have throughout the preparation process.
Innovations in Heavy Ion Radiotherapy
Bonaccorsi et al. (2024) in Cancers investigated proton and helium ion therapy versus VMAT for left-sided breast cancer. Planning studies on 10 patients showed improved target coverage with particle therapy (proton and helium) compared to VMAT, especially for internal mammary lymph nodes. 1 Particle therapy significantly reduced doses to the LAD, heart, lungs, and contralateral breast. Helium therapy demonstrated superior ipsilateral lung sparing over protons, with a predicted 2% risk of grade ≤ 2 radiation pneumonitis compared to 5% for protons and 22% for VMAT. 2 Both proton and helium reduced major coronary event risk compared to VMAT.
Wickert et al. (2022) in Cancers compared helium ion, proton, and IMRT for pediatric ependymoma. Dosimetric planning for 15 patients showed comparable target coverage across all modalities. Helium ions significantly reduced doses to critical neuronal structures by up to 39% compared to protons, and 48% compared to IMRT. Normal tissue complication probability analyses suggest helium ions could further minimize treatment-related side effects due to improved organ sparing.
Ronchi et al. (2024) in the Green Journal evaluated carbon ion radiotherapy (CIRT) for locally advanced head and neck mucosal melanoma (HNMM) in 40 patients. CIRT, at 65.6-68.8 Gy RBE in 16 fractions, achieved a 2-year local relapse-free survival (LRFS) of 84.5% and overall survival (OS) of 58.6%. LRFS was significantly better for non-recurrent status, fewer prior surgeries, and earlier treatment. Immunotherapy after relapse significantly improved median OS (17 vs 3.6 months, p<0.001). CIRT was safe, with 10% experiencing late grade ≥3 toxicity. Prospective trials are needed to explore systemic therapy combinations for improved OS.
How Much Does Heavy Ion Radiotherapy Cost?
When compared to other radiation therapies, HIR generally falls between proton therapy and standard X-ray radiation therapy in terms of cost. While both HIR and proton therapy utilize advanced technology and precision targeting, HIR facilities tend to be less numerous, which can impact accessibility and cost. Standard X-ray radiation therapy, being a more widely available and less technologically complex modality, typically incurs lower costs.
Okazaki et al. (2021) in Cancer Science compared the cost-effectiveness of carbon-ion radiotherapy (CIRT) and stereotactic body radiotherapy (SBRT) for clinical stage I NSCLC. Analyzing patients treated between 2010-2015, they calculated incremental cost-effectiveness ratios (ICERs) of 7,491,017 JPY/LY (all patients) and 3,708,330 JPY/LY (matched patients). While CIRT was deemed cost-effective, higher hospitalization and examination costs were observed. The study suggests optimizing these costs could further enhance CIRT’s cost-effectiveness.
Okazaki et al. (2024) in Advances in Radiation Oncology evaluated the cost-effectiveness of carbon-ion radiation therapy (CIRT) versus transarterial chemoembolization (TACE) for localized hepatocellular carcinoma (HCC). Using propensity score matching on 34 patients, CIRT demonstrated dominance over TACE. CIRT resulted in a higher life year (2.75 vs 2.41) and lower total cost (¥4,974,278 vs ¥5,284,524). Sensitivity analysis confirmed CIRT’s cost-effectiveness with a high acceptability rate.
Recovery of the Body After Heavy Ion Radiotherapy
Recovery following heavy ion radiotherapy (HIR) is a gradual process that varies depending on the individual, the treatment site, and the specific ions used. It involves both physical recovery and ongoing monitoring to ensure treatment success.
Physical recovery time can differ significantly among patients. Some may experience rapid recovery, while others may require more extended periods for their bodies to heal. Dietary changes are often recommended to support this recovery process. A balanced diet, rich in fruits, vegetables, and lean proteins, provides the body with the necessary nutrients for tissue repair and regeneration. Patients may also be advised to avoid certain foods or beverages that could irritate the treated area, particularly if the head and neck region was targeted.
Exercise guidelines are typically tailored to the individual’s condition and tolerance. Gentle physical activity, such as walking or light stretching, can help maintain muscle strength and flexibility. As recovery progresses, patients may gradually increase the intensity and duration of their exercise routines, always under the guidance of their healthcare team.
Long-term follow-ups are crucial for monitoring treatment success and detecting any potential late effects. These follow-up appointments typically involve regular clinical examinations, imaging studies (such as MRI or CT scans), and blood tests. These assessments allow the healthcare team to evaluate the tumor’s response to treatment, monitor for any signs of recurrence, and identify any long-term side effects, such as fibrosis or secondary cancers. These follow ups are important to ensure the patient’s long term health. Open communication between the patient and the healthcare team is essential during these follow-up periods, allowing for prompt intervention and personalized management of any emerging issues.
Writtten by Aren Karapetyan, MD
FAQ
What is heavy ion radiotherapy ?
HIR uses accelerated heavy ions, like carbon, to precisely target and destroy cancer cells.
How is HIR different from traditional X-ray therapy?
HIR delivers a concentrated radiation dose directly to the tumor, minimizing damage to healthy tissue, unlike X-ray therapy which affects broader areas.
What is the Bragg peak?
It's the point where heavy ions release the most energy, precisely within the tumor, maximizing cell damage there.
What types of cancer can be treated with HIR?
HIR effectively treats radio-resistant tumors like sarcomas, pancreatic, head and neck cancers, and recurrent tumors.
Are there side effects to HIR?
Yes, but HIR minimizes them. Short-term effects are localized, and long-term effects are monitored closely.
How long does HIR treatment take?
The treatment period is relatively short, averaging around three weeks, compared to six to seven weeks for X-ray therapy.
Is HIR more expensive than other radiation therapies?
Yes, HIR is generally more expensive than standard X-ray therapy, but can be comparable to proton therapy.
How do patients prepare for HIR?
Preparation includes imaging scans, custom immobilization devices, and simulation sessions.
What is the recovery process like after HIR?
Recovery involves dietary changes, gentle exercise, and long-term follow-up appointments.
What are the main advantages of HIR?
HIR offers precise tumor targeting, enhanced biological effectiveness, and shorter treatment durations.
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
