Blinatumomab to improve Disease free survival in Pre-B cell ALL
Vivek Subbiah shared on LinkedIn:
“Wow! BITE – Blinatumomab improves Disease free survival in Pre-B cell ALL
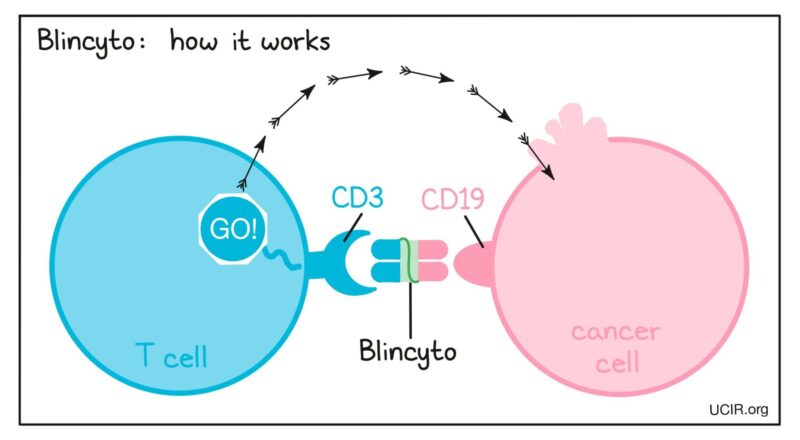
BITE = bispecific T-cell engager molecule
Blinatumomab= anti-CD19 and anti-CD3 single-chain molecule
Published NEJM Group concurrently with ASH24 meeting
Link to Original Article: Blinatumomab in Standard-Risk B-Cell Acute Lymphoblastic Leukemia in Children (AALL1731).
Authors: Sumit Gupta, Rachel E. Rau, John A. Kairalla, Karen R. Rabin, Cindy Wang, Anne L. Angiolillo, Sarah Alexander, Andrew J. Carroll, Susan Conway, Lia Gore, Ilan Kirsch, Holly R. Kubaney, Amanda M. Li, Jennifer L. McNeer, Olga Militano, Tamara P. Miller, Yvonne Moyer, Maureen M. O’Brien, Maki Okada, Shalini C. Reshmi, Mary Shago, Elizabeth Wagner, Naomi Winick, Brent L. Wood, Tara Haworth-Wright, Faraz Zaman, Gerhard Zugmaier, Sue Zupanec, Meenakshi Devidas, Stephen P. Hunger, David T. Teachey, Elizabeth A. Raetz, and Mignon L.
 ”
”
More posts featuring Vivek Subbiah.
Vivek Subbiah is the Chief of Early-Phase Drug Development at the Sarah Cannon Research Institute (USA). He is the former Executive Director of Oncology Research and former Associate Professor in the Department of Investigational Cancer Therapeutics at the MD Anderson Cancer.
He focuses on translational cancer research and the design and implementation of early-phase biomarker-driven clinical trials. His work specifically targets antibody-drug conjugates, radiopharmaceuticals, immunoconjugates, and basket trials. He has also received the Yvonne Award 2024 by OncoDaily in the “Voice of Oncology” category.
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
