On November 10, 2024, the 5-year results of the OPERA trial by D. Baron et al. were published in ESMO Annals of Oncology.
Authors: D. Baron, T. Pace Loscos, R. Schiappa, N. Barbet, E. Dost, S. Ben Dhia, S. Soltani, L. Mineur, I. Martel, S. Horn, C. Picardi, A. Stewart, E. Cotte, R. Coquard, G. Baudin, L. Evesque, A. Dhadda, A. Sun Myint, J.P. Gérard, J. Doyen

Nina Niu Sanford, Chief of Gastrointestinal Radiation Oncology at Harvard/Brigham and Women’s Hospital/Massachusetts General Hospital, shared the article on X, adding:
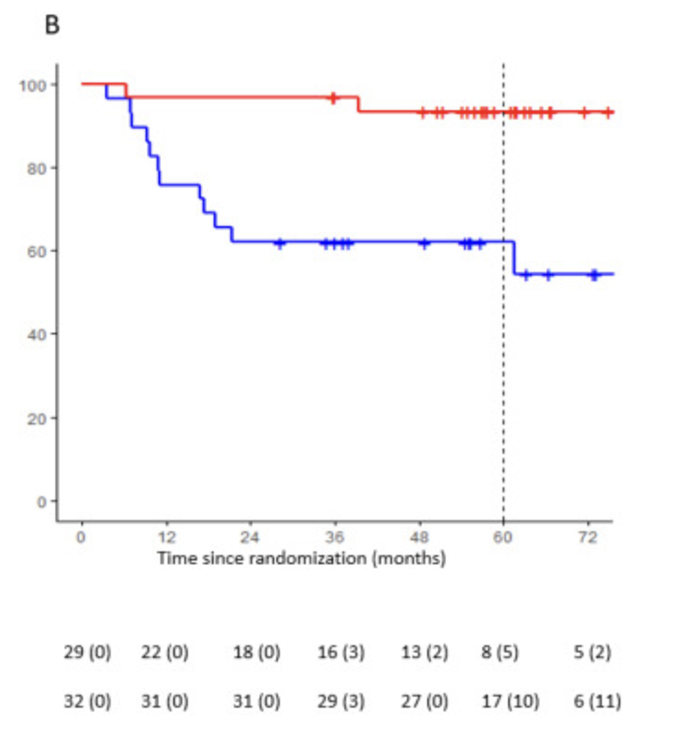
“5-year OPERA results out and continue to show higher organ pres with brachy boost: 79% vs 56% at 5 years.
With initial pub, concern re: late tox (64% brachy arm with Gr 1-2 rectal bleed), but by 3 year, bleeding resolved in 80%. No Gr 4-5 AE, LARS similar.

Both groups did well: 5-year organ preservation was 56% for chemoRT arm (without brachy).
Remember these patients only got radiosensitizing chemo, no FOLFOX. Yet results are similar to OPRA, suggesting this trial really did enroll lower risk patients.
Worth noting the really outstanding results for tumors smaller than 3 cm: in brachy boost arm, 93% 5-year OP and only 3% regrowth rate. So, most of these patients can be cured/keep their rectums with essentially RT alone.
In contrast, there was no SS improvement in organ pres with brachy boost for tumors >3 cm: 57% vs. 67% (p=0.17), suggesting larger tumors need additional therapy (such as chemo).
One caveat is that local excision was an option for patients with cCR or nCR and also for those experiencing local regrowth – and I believe these patients were still counted as having organ pres. Most centers manage local regrowth with TME.
Lastly, 3 of 28 cases of local regrowth occurred beyond 3 years, showing importance of continued surveillance (in OPRA, 99% of regrowths were caught in 3 years).
The study first came out in 2023.”
Cathy Eng, Co-Leader of the Gastrointestinal Cancer Research Program at the Vanderbilt-Ingram Cancer Center, shared this post on X, adding:
“Glad to see the updated results from OPERA rectal trial.
Nina Niu Sanford, the Papillon RadOnc technique is rarely used even in France where it was created. Are you suggesting rad/oncs will revise their approach to rectal cancer given this data?”
Nina Niu Sanford is an Assistant Professor and Chief of Gastrointestinal Radiation Oncology at Harvard/Brigham and Women’s Hospital/Massachusetts General Hospital. She specializes in treating gastrointestinal cancers and actively participates in clinical trials combining high-dose radiation therapy with immunotherapy. Additionally, she researches healthcare access disparities and conducts pan-cancer outcomes research using large databases.
Cathy Eng is a Professor of Medicine, Hematology and Oncology, and is the Co-Director of GI Oncology, Associate Director of Strategic Relations and Research Partners and Co-Leader of the Gastrointestinal Cancer Research Program at the Vanderbilt-Ingram Cancer Center. She also holds the David H. Johnson Endowed Chair of Surgical and Medical Oncology. Dr. Eng was previously a Professor of Medical Oncology at The University of Texas MD Anderson Cancer Center.
Her primary clinical research interests include clinical trials involving innovative drugs for the treatment of colorectal, anal, and appendiceal cancers. She has a specific interest in young colorectal cancer patients as well as the role of immunotherapy in HPV-associated cancers. Nationally, Dr. Eng has served in multiple leadership roles for ASCO, ASCO GI, ECOG, and the NCI Rectal/Anal Task Force. She has most recently been chosen to serve as the Vice-Chair for the SWOG GI Committee and the NCI GI Steering Committee.


