Triparna Sen, Associate Professor at Icahn School of Medicine at Mount Sinai, shared a post on X about recent paper she and colleagues co-authored, titled “Lurbinectedin sensitizes PD-L1 blockade therapy by activating STING-IFN signaling in small-cell lung cancer” published on Cell Reports Medicine.
Authors: Subhamoy Chakraborty, Utsav Sen, Kedwin Ventura, Vrinda Jethalia, Charles Coleman, Subhasree Sridhar, Avisek Banerjee, Hilal Ozakinci, Yazhini Mahendravarman, Konrad Snioch, Elisa de Stanchina, Misty D. Shields, Lewis E. Tomalin, Deniz Demircioglu, Theresa A. Boyle, Anna Tocheva, Dan Hasson, Triparna Sen.

“We’re thrilled to share our latest study, “Lurbinectedin sensitizes PD-L1 blockade therapy by activating STING-IFN signaling in small-cell lung cancer,” now published in Cell Reports Medicine.
This study provides critical preclinical evidence showing that lurbinectedin reshapes the immune microenvironment in SCLC—helping immunotherapy work better.
This study is especially timely given the recent clinical progress in this space:
- The LUPER study demonstrated that lurbinectedin plus pembrolizumab showed promising efficacy in relapsed SCLC, particularly for platinum-sensitive patients.
- The press release that in the IMForte Phase 3 trial, lurbinectedin + atezolizumab shows statistically significant improvement in overall survival (OS) and progression-free survival (PFS) as maintenance therapy in extensive-stage SCLC.
These clinical results are encouraging—and our study explains why this combination works so well.
SCLC remains one of the most aggressive and challenging cancers to treat.
For decades, chemotherapy was the only viable option, and while the addition of immune checkpoint inhibitors (ICIs) (like anti-PD-L1 therapies) has helped, the benefits have been modest and short-lived for most patients. The problem? SCLC is an “immune-cold” tumor—it lacks the T cell infiltration needed for immunotherapy to work effectively.
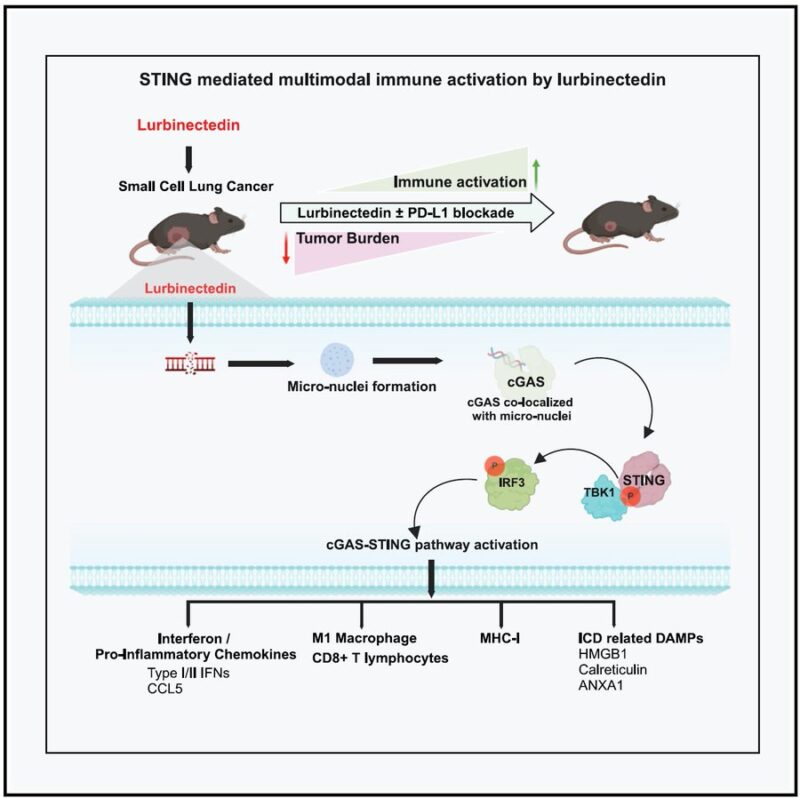
Our research shows that lurbinectedin doesn’t just kill cancer cells—it also “reprograms” the immune microenvironment, making tumors more susceptible to PD-L1 blockade therapy.
Our study reveals:
- Lurbinectedin activates the STING-IFN pathway, a critical immune surveillance system that boosts interferon signaling and alerts the immune system to the presence of tumors.
- It increases MHC-I expression, making cancer cells more visible to immune cells.
- It enhances CD8+ T cell infiltration, ensuring that more cancer-fighting immune cells reach the tumor site.
- It shifts macrophages from an immunosuppressive (M2) state to a pro-inflammatory (M1) state, further supporting a more active anti-tumor immune response.
In short, lurbinectedin “primes” SCLC tumors to respond to immunotherapy, helping checkpoint inhibitors like atezolizumab and pembrolizumab work more effectively. For SCLC patients, this could mean more durable responses and new therapeutic possibilities.
Most interestingly-
- RNA STEP analysis shows upregulation of DNA-damage-related genes & immune markers (T cells, B cells, macrophages) post-lurbinectedin treatment in pre- vs post-lurbinectedin treated SCLC clinical samples.
- MHC-I/II and PD-L1 gene expression increased, enhancing immune recognition of tumors.
- Multiplex immunofluorescence confirms more CD8+ T cells infiltrating lung tumors and CD19+/CD138+ antigen-presenting cells.
Lymph nodes of treated patients show: More cytotoxic CD8+ T cells in metastases (liver and lymph nodes),Higher 4-1BB+ T cells → stronger co-stimulatory response, Increased antigen-presenting cells (CD19+/CD138+) in tumor stroma.
Exciting time SCLC research and treatment.
- Our study provides the mechanistic foundation for lurbinectedin + immunotherapy combinations.
- LUPER shows real-world efficacy in relapsed patients.
- IMForte press release shows promise.
Together, these findings suggest that lurbinectedin is an immunotherapy enhancer that could expand treatment options for patients with SCLC.
This work was led by my amazing Subhamoy Hakraborty.
A huge thank you to my incredible coauthors and funders! This paper was a multi-institution collaboration and wouldn’t have been possible without the team’s expertise and support. Research thrives on teamwork and funding, and I’m grateful to be part of such an amazing network.”