Lena Wirth, Immunobiology Graduate Student at Yale University, shared a post on X about a paper she co-authored with colleagues published in Cancer Discovery:
Authors: Miya Hugaboom, Lena Wirth, Kelly Street, Catherine Wu, David Braun et al.

“Excited to share our latest work published in Cancer Discovery. Led by Miya Hugaboom, Kelly Street, we show that the presence of tertiary lymphoid structures and exhausted tissue-resident T cells determines clinical response to PD-1 blockade in RCC patients.
Huge thanks to all the investigators listed below for their hard work in making this study possible – this was a true team effort! Most importantly, thank you to the patients and their families, and the senior authors.
ICI targeting PD-1/PD-L1 have transformed RCC treatment, yet most patients lack durable responses. While transcriptomic studies have advanced our understanding of the RCC TME, the role of individual cell populations in ICI resistance remains unclear, highlighting the need to define determinants of response and resistance.
We analyzed bulk and single-cell transcriptomes of RCC tumors from treatment-naïve patients in the HCRN GU16-260 trial (Hoosier Cancer) before and/or at resistance to nivo.
Bulk data showed enriched B cell-related genes and TLS signatures in responders, confirmed by multiplex IF in 19 patients.
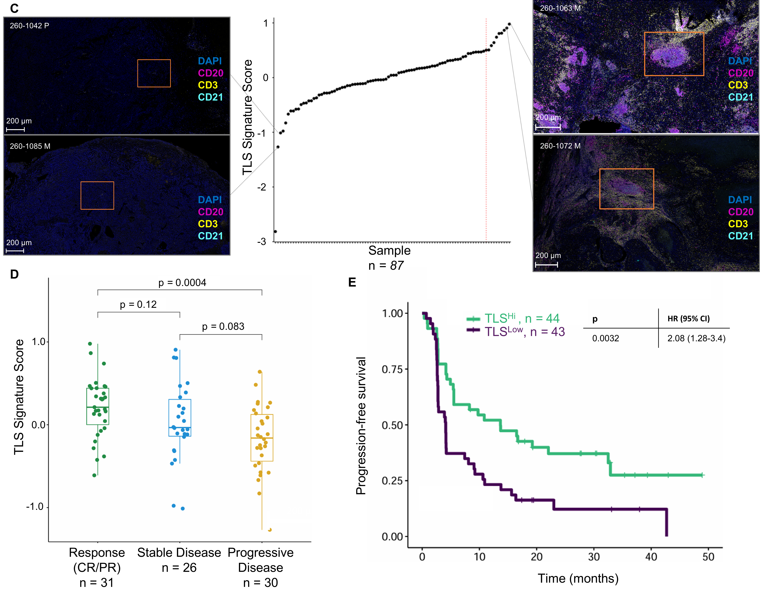
Single-cell analysis of 28,268 T cells identified a tissue-resident like ZNF683+ SLAMF7+ exhausted CD8+ population associated with worse objective response and PFS.
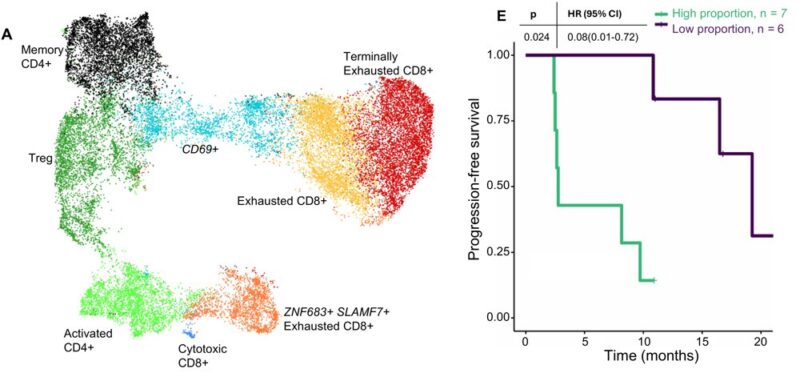
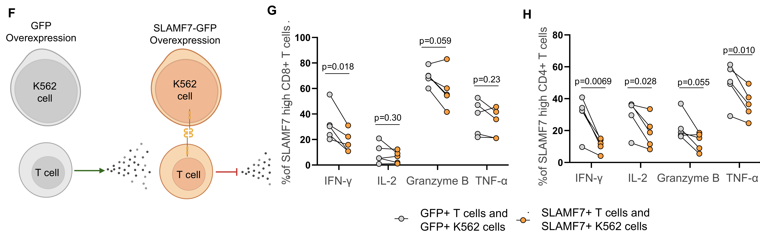
Using established gene signatures, these cells were inferred to have high tumor specificity, despite their association with resistance. We further investigated SLAMF7 as a potential driver of ICI resistance.
Functional studies showed that SLAMF7 activation reduced pro-inflammatory cytokine production and proliferation in human CD4+ and CD8+ T cells in vitro, suggesting a role in driving T cell dysfunction.
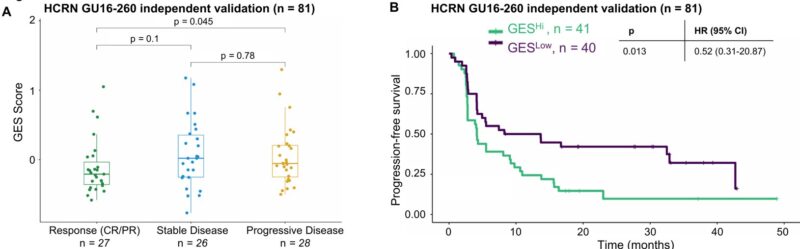
Using matched bulk and scRNA-seq, we generated a ZNF683+ SLAMF7+ GES specific to tissue-resident exhausted cells. In 81 independent HCRN trial samples, the GES was higher in PD vs. CR/PR and associated with worse clinical outcomes.
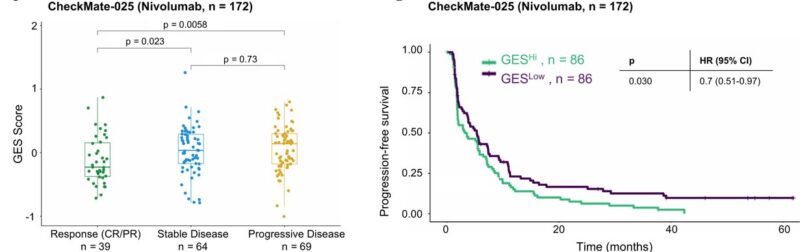
Further validation in the external CheckMate-025 RCC cohort showed the same trend in the nivo arm – higher GES in PD and worse PFS/OS. However, the GES score showed no associations with outcomes in the everolimus arm, highlighting the specificity to PD-1 blockade.
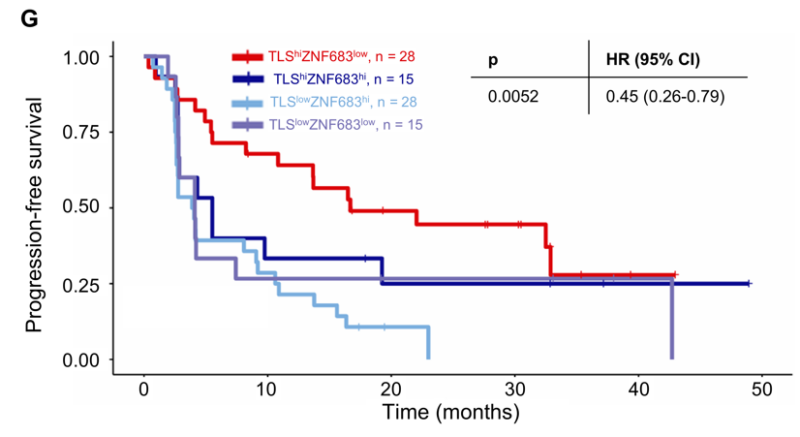
Finally, we examined how TLS and tissue-resident exhausted CD8+ T cells impacted outcomes with nivo. Patients were grouped by median GES split: TLS hi/ZNF683 lo, TLS hi/ZN683 hi, TLS lo/ZNF683 lo, TLS lo/ZNF683 hi. Patients with TLS hi/ZNF683 lo had improved PFS and OS.
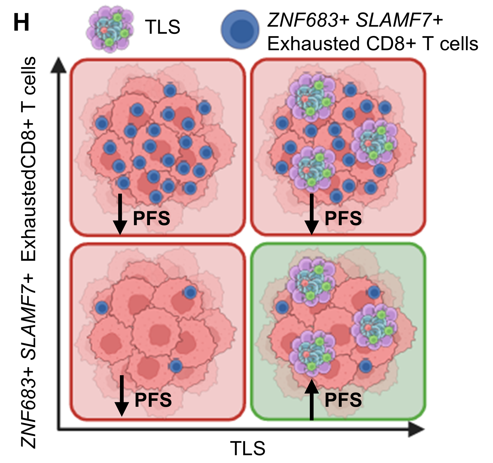
Overall, we identify an interplay in the RCC TME between TLS and ZNF683+ SLAMF7+ exhausted CD8+ T cells that contribute to clinical outcomes with PD-1 blockade. This may help predict response to current therapies and inform strategies to overcome resistance to PD-1 ICI.
Finally, we would like to thank the DOD CDMRP program and NCI for their support, and the Cancer Discovery Editors and Reviewers for their exceptional guidance through the submission.”
More posts about RCC.


