Laura Bukavina, Urologic Oncologist at University Hospitals Cleveland Medical Center, shared a post on X/Twitter:
“As we gear up for today’s webinar, let’s dive into the topic of Non-muscle-invasive Bladder Cancer (NMIBC), starting with an understanding of BCG unresponsive disease, exploring the progression of the disease if it remains untreated, FDA regulations, examining the various trials in NMIBC. This area is constantly evolving, so you can read below to deepen your knowledge.
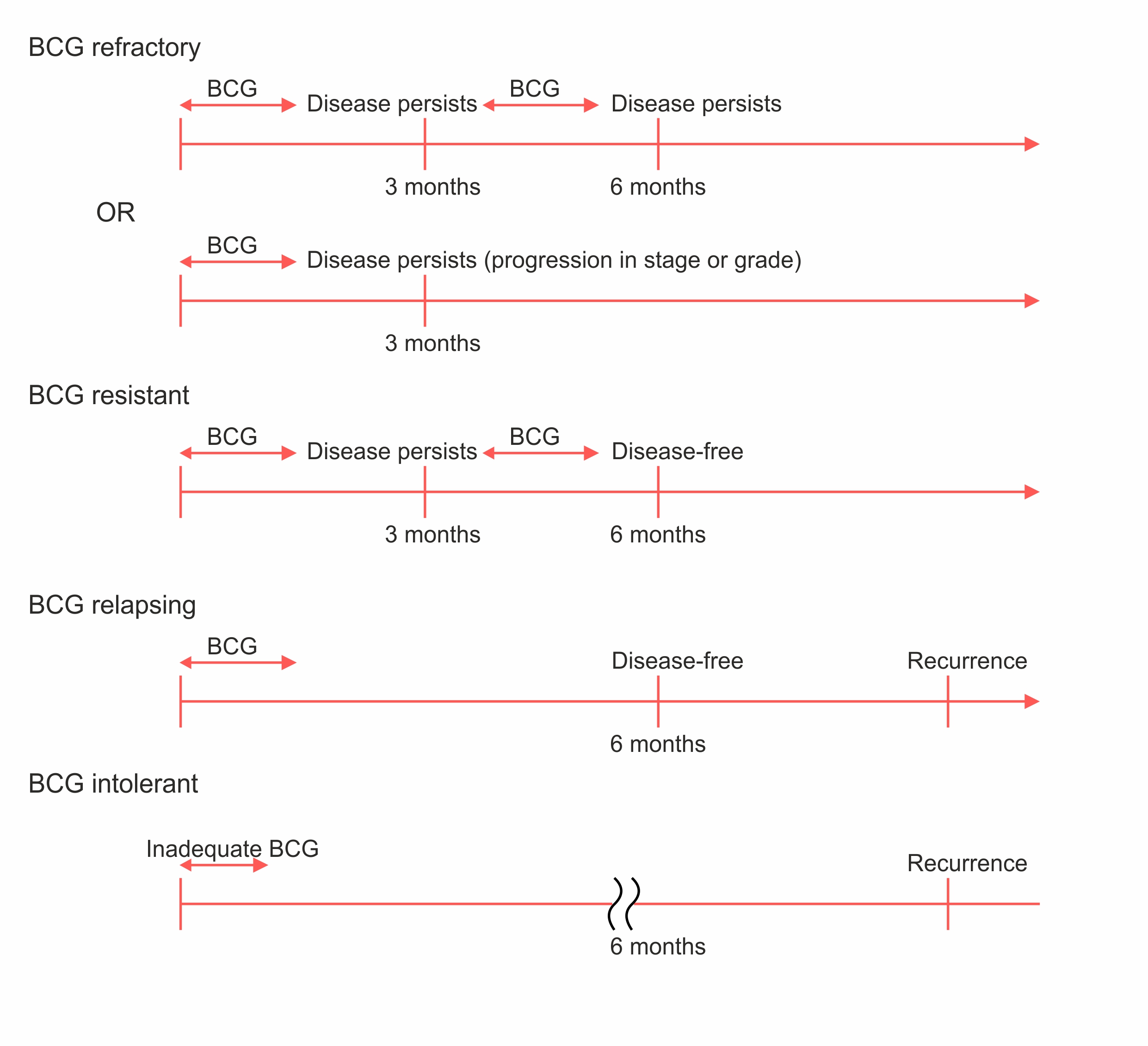
Understanding NMIBC: Key Categories:
- Refractory: High-grade disease persists at 6 months despite BCG therapy. Includes any stage/grade progression by 3 months.
- Relapsing: High-grade recurrence after disease-free state at 6 months post-BCG.
- Intolerant: Inability to complete BCG due to toxicity.
- Unresponsive: Encompasses both BCG refractory and relapsing cases. This high-risk group needs alternatives to BCG.
Among the BCG relapse patients, those with tumor recurrence within 6 months(CIS/T1) of the last BCG exposure have similar outcomes as those who are BCG refractory. When evaluating the results of agents in single-arm studies, these groups are the most of interest.
Timeframe for Finding Alternatives to Cystectomy in BCG Unresponsive Patients: When a patient is classified as BCG unresponsive, the clock starts ticking. We have approximately 12 months, or the duration equivalent to 2-4 Trans urethral removal of bladder tumour (TURBTs), to identify and implement an effective alternative therapy.
Defining Adequate BCG Therapy in Urology: 1. Minimum 5/6 doses in the initial induction course, plus at least two out of three doses in maintenance therapy. 2. Alternatively, at least 5/6 doses in the initial induction course, followed by 2/6 doses in a second induction course.
As we dive into trials you may notice many are single-arm as FDA justifies this approach, stating “Single-arm trials are suitable given the lack of effective medical therapies available, with radical cystectomy as the only alternative”
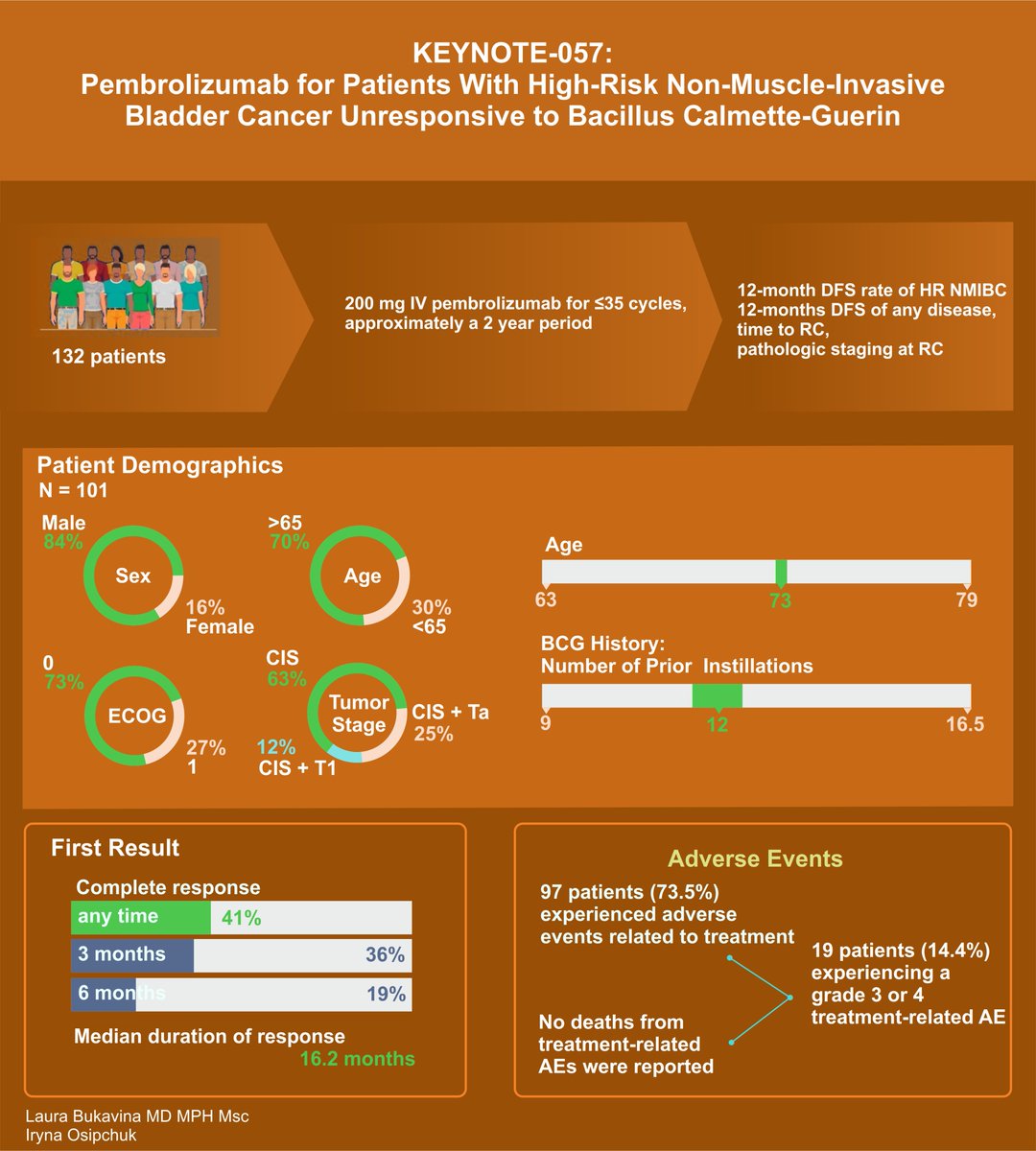
First on our list: KEYNOTE-057 Study Highlights in Bladder Cancer Treatment. Pembrolizumab Key findings:
- 41% CR rate
- Median duration of CR: 16.2 months.
- 46% of initial responders had responses lasting 12 months or more.
- Grade 3 or 4 treatment-related adverse events in 1% of patients.
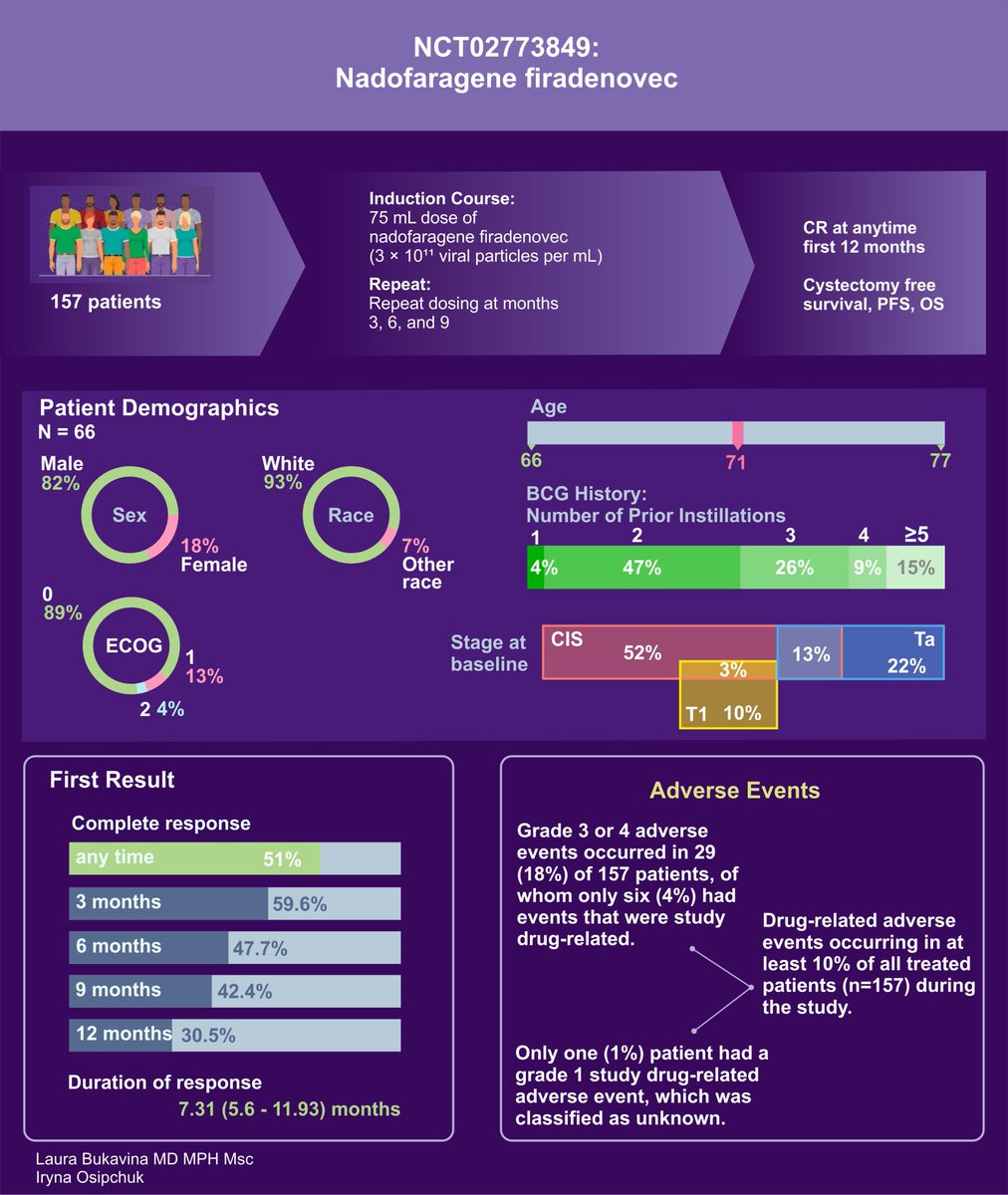
Moving on to Key Outcomes from Nadofaragene Firadenovec IFN adenovirus therapy Study in BCG-Unresponsive NMIBC:
- 53.4% of patients achieved CR at 3 months
- 12 months, 45.5% maintained this response
- High-grade Ta or T1 patients, 72.9% were high-grade recurrence-free at 3 months; 43.8% at 12 months
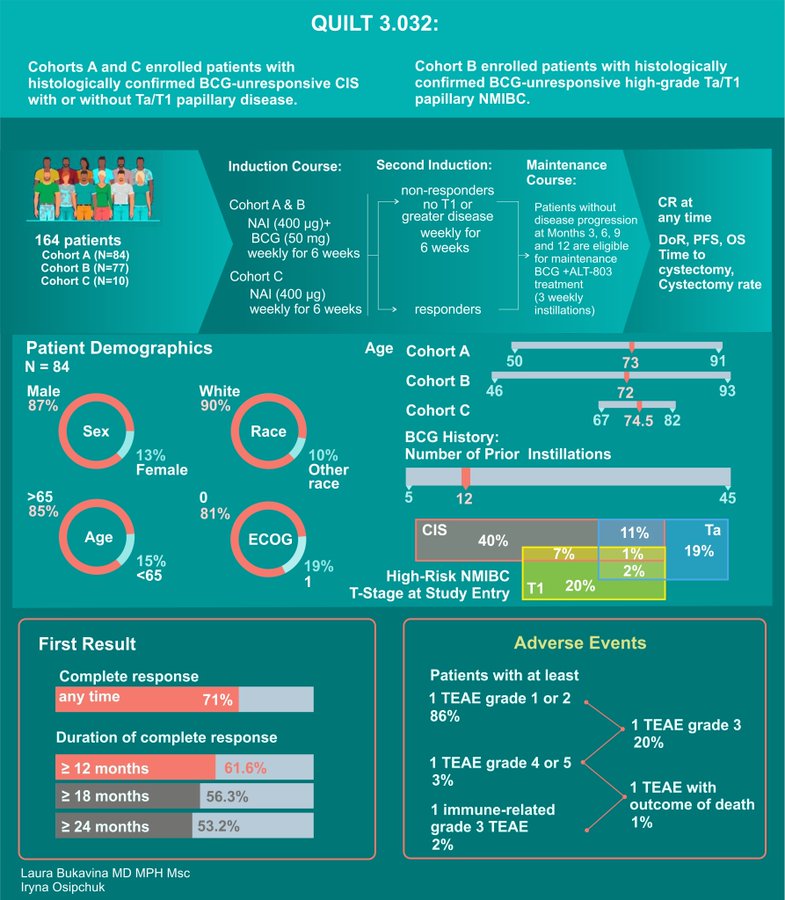
IL-15 Superagonist N803 QUILT 3.032 trial with BCG with phenomenal results.
- 71% CR
- Sustained median response duration of 26.6 months
- Remarkable 89.2% avoidance of cystectomy at 24 months in patients with CR
- 100% disease-specific survival rate at 24 months.
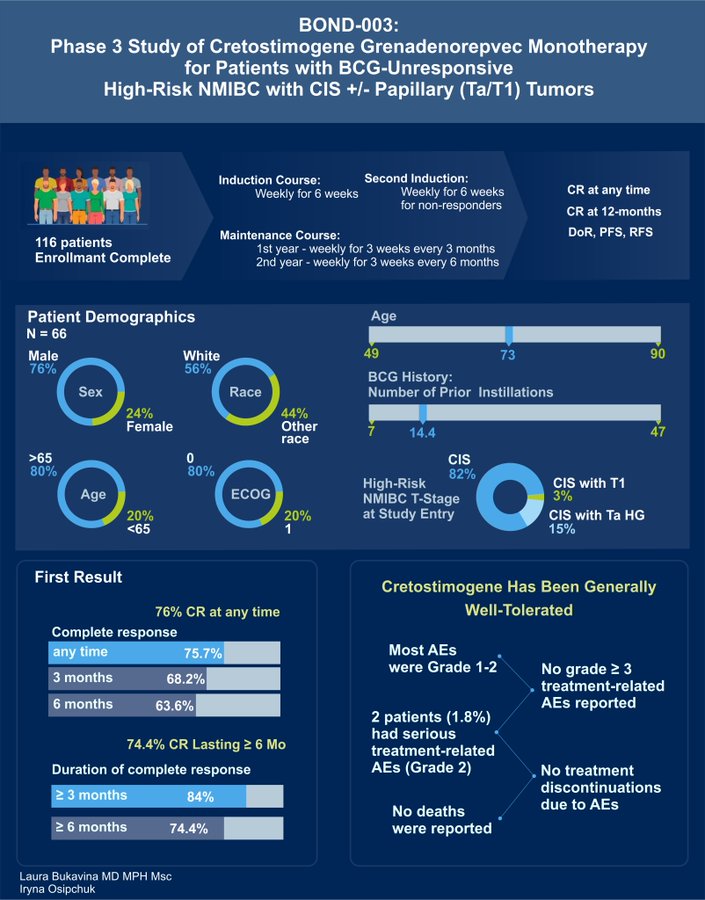
BOND-003 Oncolytic Immunotherapy Cretostimogene monotherapy as presented by Mark Tyson and Roger Li:
- 75.7% CR anytime, with 74.4% of patients with sustained response greater than 6 months
- No grade 3> AE and no treatment discontinuation
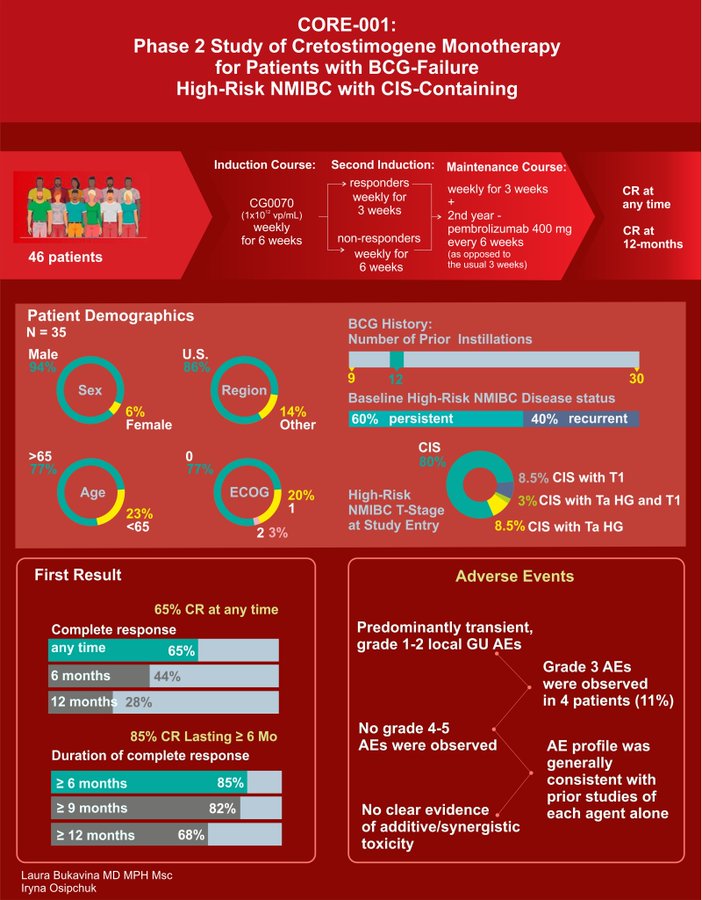
CORE-001 combination Cretostimogene plus systemic Pembro presented by Roger Li with similarly great results including 85% CR at any time, with 68% CR at 12 months with 1 treatment related discontinuation and 0% Grade 3 TRAE in phase II trial of 35 patients.
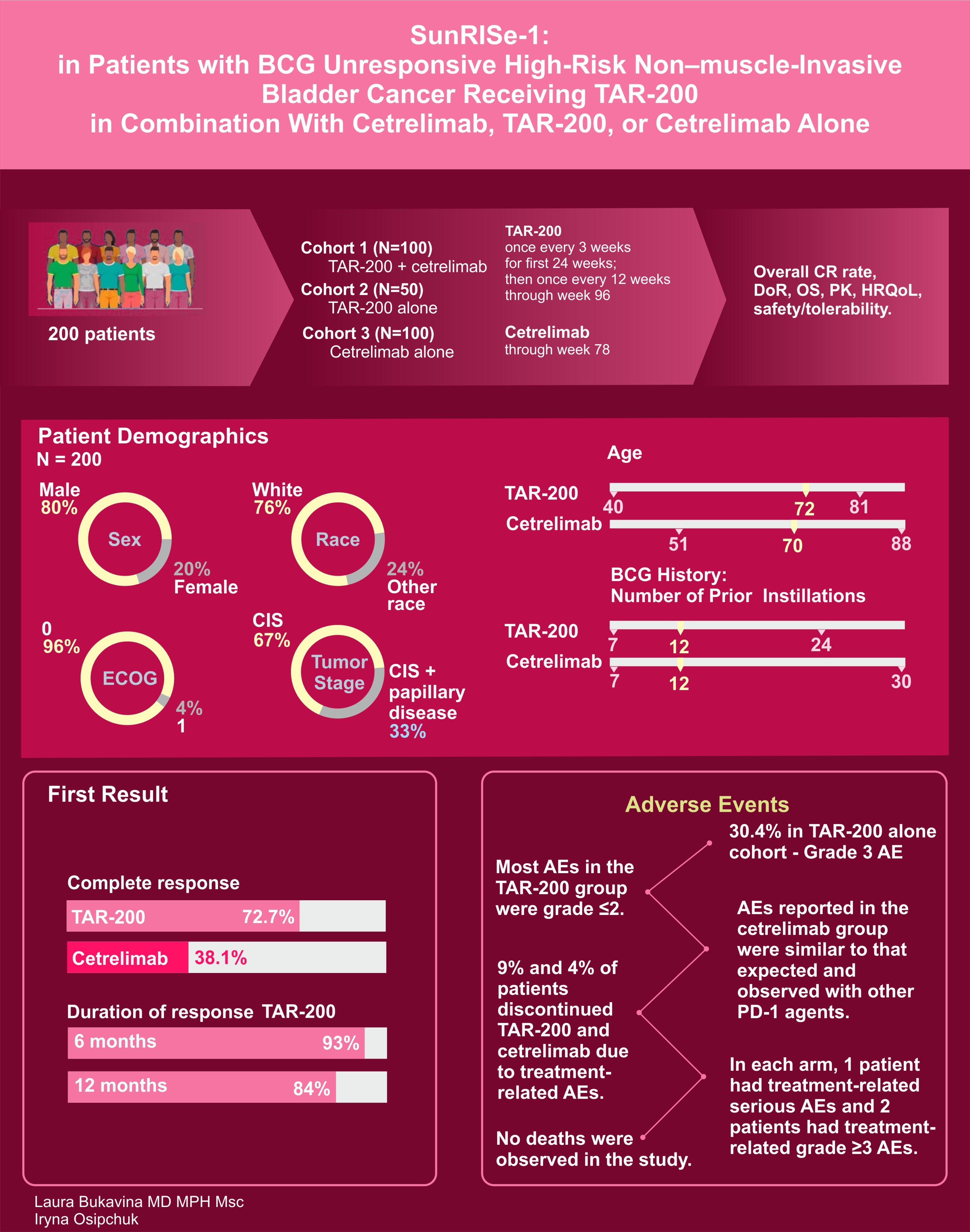
And of course we cannot forget about SUNRISE-1 of TAR-200 with and without Cetrelimab with
- 72.7% of TAR-200 alone cohort patients experiencing CR with only 38.1% with cetrelimab alone (above 30% threshold).
- Unlike many others, 30.4% of cohort 2 (TAR200 alone) patients did experience Group 3 or higher AE
- 9% treatment discontinuation in TAR 200 alone.
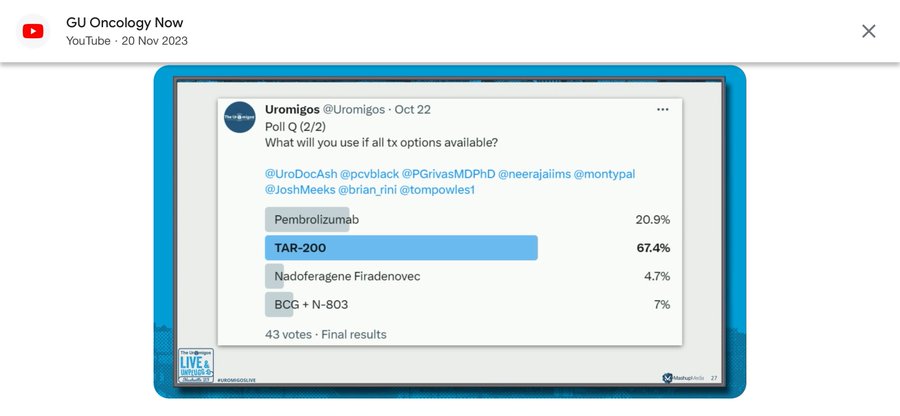
Fascinatingly, as Uromigos pointed out, it seems that most physicians, given the option, would opt for TAR-200, with pembrolizumab coming in as the runner-up. Given the data we’ve just reviewed, would you concur with this preference, or has the new information swayed your opinion? Share your thoughts!”
Source: Laura Bukavina/X