Zongertinib (BI 1810631), an oral, irreversible HER2-selective tyrosine kinase inhibitor, has emerged as a promising therapeutic option for patients with previously treated HER2-mutant non–small-cell lung cancer (NSCLC). Unlike earlier pan-HER inhibitors, zongertinib is designed to selectively target HER2 while sparing EGFR, potentially reducing the incidence of EGFR-related toxicities such as diarrhea and rash.
HER2 mutations, occurring in approximately 2–4% of NSCLCs, are predominantly found within the tyrosine kinase domain, particularly exon 20 insertions. Despite the availability of trastuzumab deruxtecan as an FDA-approved HER2-targeted therapy, treatment options remain limited, and concerns about interstitial lung disease persist.
The recently published results from the Beamion LUNG-1 phase 1b trial, reported in the New England Journal of Medicine, provide new insights into the efficacy and safety profile of zongertinib in this challenging patient population. The trial demonstrated a 71% confirmed objective response rate, durable responses, and a manageable safety profile among heavily pretreated patients with HER2-mutant NSCLC.
Title: Zongertinib in Previously Treated HER2-Mutant Non–Small-Cell Lung Cancer
Authors: John V. Heymach, M.D., Ph.D., Gerrina Ruiter, M.D., Ph.D., Myung-Ju Ahn, M.D., Nicolas Girard, M.D., Ph.D., Egbert F. Smit, M.D., Ph.D., David Planchard, M.D., Ph.D., Yi-Long Wu, M.D., Byoung Chul Cho, M.D., Ph.D., Noboru Yamamoto, M.D., Ph.D., Joshua K. Sabari, M.D., Yanqiu Zhao, M.D., Hai-Yan Tu, M.D., Kiyotaka Yoh, M.D., Ernest Nadal, M.D., Ph.D., Behbood Sadrolhefazi, M.D., Maren Rohrbacher, M.D., Ph.D., Ute von Wangenheim, Ph.D., Sabina Eigenbrod-Giese, M.D., Ph.D., and Jon Zugazagoitia, M.D., Ph.D., for the Beamion LUNG-1 Investigators
Background
Approximately 2–4% of non–small-cell lung cancers (NSCLC) harbor HER2 (ERBB2) mutations, predominantly in the tyrosine kinase domain, particularly exon 20. Trastuzumab deruxtecan remains the only FDA-approved HER2-directed therapy for these patients, but its use is associated with risks such as interstitial lung disease. Pan-HER tyrosine kinase inhibitors (TKIs) have shown limited efficacy and considerable toxicity. Zongertinib (BI 1810631), a novel, oral, irreversible, HER2-selective TKI sparing EGFR, was developed to address these challenges. Early-phase studies demonstrated encouraging antitumor activity with a manageable safety profile.
Methods
The Beamion LUNG-1 study is a multicohort, phase 1a–1b trial evaluating zongertinib in HER2-altered tumors. This primary report focuses on patients with previously treated HER2-mutant advanced or metastatic NSCLC across three cohorts:
- Cohort 1: Tyrosine kinase domain mutations without prior HER2-directed therapy
- Cohort 5: Tyrosine kinase domain mutations with prior HER2-directed antibody–drug conjugate exposure
- Cohort 3: Non–tyrosine kinase domain mutations
Eligible patients had histologically confirmed NSCLC, prior platinum-based chemotherapy, measurable disease per RECIST v1.1, and ECOG performance status 0–1.
The primary endpoint was the confirmed objective response rate (ORR) assessed by independent review (cohorts 1 and 5) or investigator assessment (cohort 3). Secondary endpoints included duration of response (DoR) and progression-free survival (PFS).
Study Design
Initially, patients in cohort 1 were randomized to 120 mg or 240 mg zongertinib daily. Interim analysis supported the selection of 120 mg as the optimal dose based on efficacy and reduced adverse events. All subsequently enrolled patients received 120 mg once daily in 21-day cycles until disease progression or unacceptable toxicity.
The sample size was powered to detect meaningful improvements over historical benchmarks (≤30% ORR for cohort 1, ≤25% for cohort 5).
Results
A total of 132 patients were enrolled in cohort 1, 39 in cohort 5, and 25 in cohort 3 between March 2023 and November 2024 across 74 sites globally.
In cohort 1 (n=75; 120 mg dose), the confirmed ORR was 71% (95% CI, 60–80; P<0.001), including 7% complete responses and 64% partial responses.
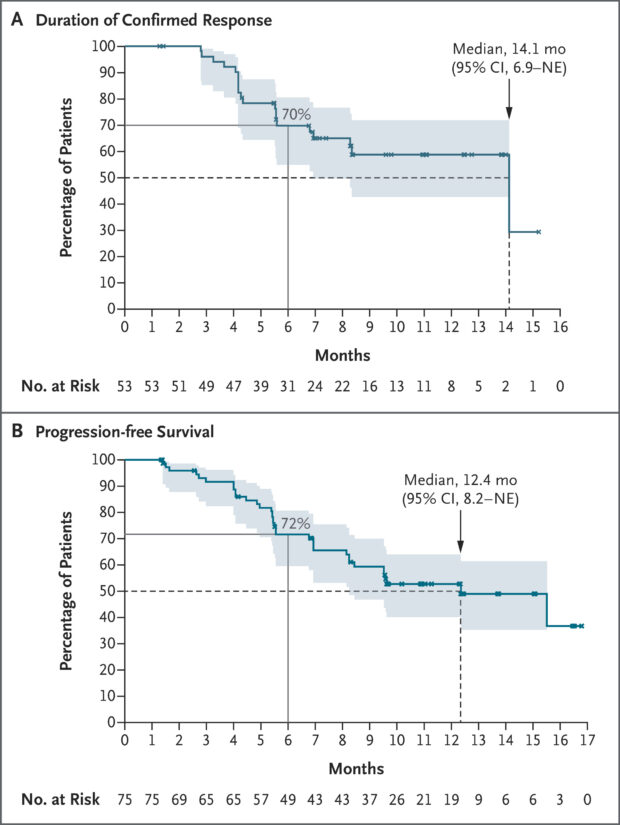
- The median DoR was 14.1 months (95% CI, 6.9–NE).
- The median PFS was 12.4 months (95% CI, 8.2–NE).
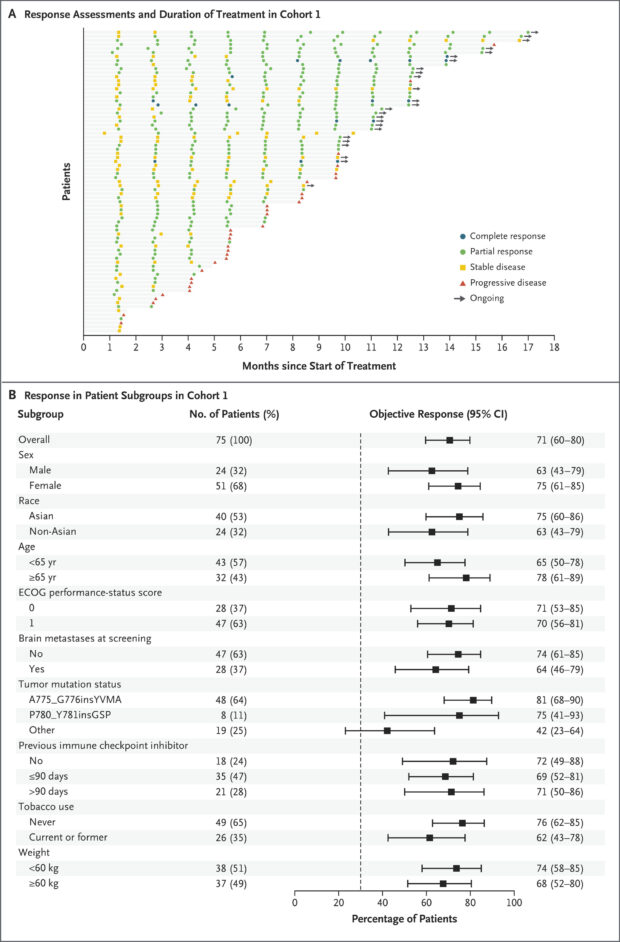
Responses were consistent across subgroups, including patients with brain metastases (ORR 64%).
In cohort 5 (n=31), comprising patients previously treated with HER2-directed ADCs, the confirmed ORR was 48% (95% CI, 32–65), with a median DoR of 5.3 months and median PFS of 6.8 months.
In cohort 3 (n=20), representing non-tyrosine kinase domain mutations, the ORR was 30% (95% CI, 15–52).
Regarding safety:
- Grade ≥3 drug-related adverse events occurred in 17% of cohort 1 patients, primarily elevated liver enzymes.
- Diarrhea (any grade) occurred in 56%, but only 1% were grade 3.
- Rash (any grade) was reported in 33%; no grade ≥3 rashes occurred.
- No cases of drug-related interstitial lung disease were observed across all cohorts.
- Discontinuations due to adverse events were rare (3%).
Key Findings
- High ORR of 71% with zongertinib 120 mg in previously treated HER2-mutant NSCLC.
- Durable responses with median DoR of 14.1 months and median PFS of 12.4 months.
- Manageable toxicity profile, with mostly grade 1–2 diarrhea and rash; minimal serious toxicity.
- Promising intracranial activity, with a 41% intracranial ORR in evaluable patients with brain metastases.
- Zongertinib demonstrated activity even after prior treatment with trastuzumab deruxtecan.
- Selectivity for HER2 over EGFR contributed to lower rates of EGFR-related toxicities compared to pan-HER TKIs.
Key Takeaway Messages
The Beamion LUNG-1 study shows that zongertinib at 120 mg daily provides a high response rate, long duration of disease control, and an excellent safety profile in patients with previously treated HER2-mutant advanced NSCLC. Notably, its efficacy extends to patients with brain metastases and those previously exposed to HER2-directed ADCs.
Given its favorable comparison to existing therapies like trastuzumab deruxtecan, zongertinib has the potential to become a key therapy in HER2-mutant NSCLC, pending confirmation from phase 3 trials.
Zongertinib offers a highly active, well-tolerated, oral treatment option for patients with HER2-mutant NSCLC.
- Its selective inhibition of HER2 and sparing of EGFR may represent a significant advancement over existing pan-HER TKIs.
- Durable responses and low toxicity make zongertinib a potential candidate for both systemic and brain-metastatic disease control.
- This agent may expand options beyond antibody–drug conjugates, especially for patients progressing after trastuzumab deruxtecan.
- The ongoing Beamion LUNG-2 phase 3 study will determine whether zongertinib can move to the first-line setting.
You can read the full article here.
Written by Sona Karamyan, MD