Daiichi Sankyo and Astrazeneca issued a press release on March 6, 2025, about the latest outcomes of the DESTINY-Gastric04 trial, which show promise as a breakthrough for patients with HER2-positive gastric cancer who have progressed after first-line treatment.
A press release: ENHERTU® Demonstrates Statistically Significant and Clinically Meaningful Improvement in Overall Survival in Patients with HER2-Positive Metastatic Gastric Cancer

Metastatic gastric cancer, particularly HER2-positive gastric cancer, remains a major global health challenge. This aggressive cancer is characterized by poor prognosis, with a 5-year survival rate of only 5-10% in advanced stages. Before the advent of ENHERTU® (trastuzumab deruxtecan), no HER2-targeted therapies had demonstrated a survival benefit in the second-line metastatic setting after progression on trastuzumab-based treatments. Recent clinical developments, particularly with antibody-drug conjugates (ADCs) like ENHERTU, have begun to shift the landscape of treatment options for HER2-positive gastric cancer. ENHERTU, developed by Daiichi Sankyo and AstraZeneca, has already shown efficacy in various cancers, and the results of the DESTINY-Gastric04 trial provide significant insight into its potential for improving outcomes in second-line metastatic gastric cancer.
What Drug is Trastuzumab Deruxtecan and How Does it Work?
Trastuzumab Deruxtecan (ENHERTU®) is an innovative HER2-directed antibody-drug conjugate (ADC) developed by Daiichi Sankyo in collaboration with AstraZeneca.

Trastuzumab Deruxtecan consists of a HER2-targeted monoclonal antibody (trastuzumab) attached to a cytotoxic chemotherapy agent (deruxtecan, a topoisomerase I inhibitor) via a stable linker. The antibody component of the drug binds specifically to HER2 receptors on tumor cells. Once bound, the drug is internalized by the cancer cell, releasing the potent chemotherapy agent (deruxtecan), which interferes with DNA replication and induces cell death.
This dual mechanism—targeting HER2 for specific binding and delivering a potent chemotherapy agent directly into the cancer cell—is what makes trastuzumab deruxtecan a highly effective ADC. This specificity helps reduce damage to normal cells, minimizing side effects compared to traditional chemotherapy.
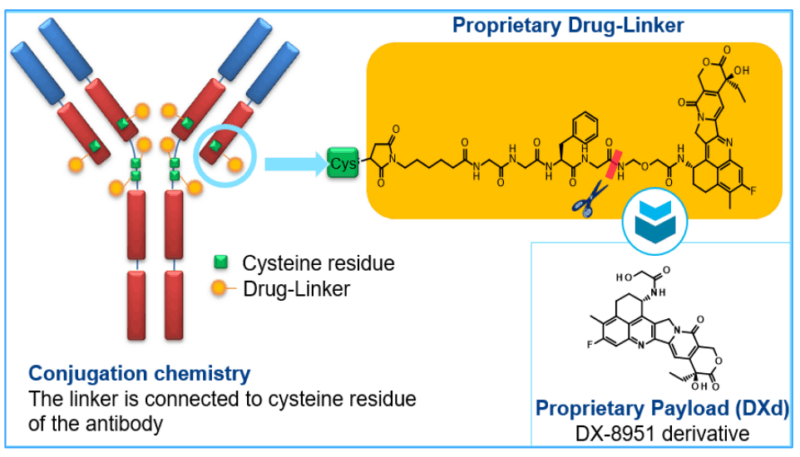
The DESTINY clinical trials are a series of pivotal studies evaluating the efficacy and safety of trastuzumab deruxtecan (T-DXd, ENHERTU®) in patients with HER2-positive and HER2-low cancers across different tumor types. These trials have demonstrated significant improvements in progression-free survival (PFS) and overall survival (OS) compared to standard therapies, leading to regulatory approvals worldwide.
Daiichi Sankyo and Astrazeneca recently issued a press release about the latest outcomes of the DESTINY-Gastric04 trial, which show promise as a breakthrough for patients with HER2-positive gastric cancer who have progressed after first-line treatment.
DESTINY-Gastric04 trial
The DESTINY-Gastric04 trial is a global, randomized, open-label, phase 3 study evaluating the efficacy and safety of ENHERTU (6.4 mg/kg) in comparison to standard chemotherapy, specifically ramucirumab and paclitaxel, in patients with HER2-positive metastatic gastric or gastroesophageal junction (GEJ) adenocarcinoma.
Methods
In DESTINY-Gastric04 trial the patient population included individuals with disease progression on or after first-line treatment with a trastuzumab-containing regimen. The primary endpoint of the trial was overall survival (OS), with secondary endpoints assessing progression-free survival (PFS), objective response rate (ORR), duration of response (DOR), disease control rate (DCR), and safety.
The study enrolled 494 patients from regions including Asia, Europe, and South America, reflecting a diverse global cohort. The interim analysis was conducted after a predefined number of events in the OS analysis, and an independent data monitoring committee reviewed the data to make recommendations on unblinding the trial.
Study Design
The DESTINY-Gastric04 study design focused on comparing the efficacy of ENHERTU against the established chemotherapy regimen of ramucirumab and paclitaxel in second-line treatment of metastatic HER2-positive gastric or GEJ cancer.
Patients were randomized to receive either ENHERTU (6.4 mg/kg) or the combination of ramucirumab (8 mg/kg) and paclitaxel (80 mg/m²). The trial aimed to provide robust data on whether ENHERTU could outperform the chemotherapy regimen in terms of improving OS and other clinical outcomes.
The study was designed to include both efficacy assessments, such as OS and PFS, and safety assessments to ensure a comprehensive evaluation of the treatment’s benefit-risk profile. The key inclusion criteria for the trial were patients with unresectable or metastatic HER2-positive gastric or GEJ adenocarcinoma who had experienced disease progression after prior treatment with a trastuzumab-containing regimen.
Results
At the planned interim analysis, ENHERTU demonstrated a statistically significant and clinically meaningful improvement in overall survival (OS) compared to the ramucirumab and paclitaxel regimen. The data was so compelling that the Independent Data Monitoring Committee (IDMC) recommended unblinding the trial early. Although full statistical details were not disclosed in the topline results, the interim data confirmed that ENHERTU improved OS compared to standard chemotherapy, a landmark finding in the treatment of HER2-positive metastatic gastric cancer.
In addition to OS, secondary endpoints including PFS, ORR, and DCR also favored ENHERTU. These results highlight ENHERTU’s potential to provide significant clinical benefit, extending survival for patients in this difficult-to-treat setting. Importantly, the safety profile of ENHERTU observed in the trial was consistent with previous studies, reinforcing its well-established tolerability.
The trial’s global scope, with 494 patients enrolled across multiple continents, provides robust evidence of the efficacy of ENHERTU in diverse populations. These results align with earlier findings in Phase 2 trials, further solidifying ENHERTU’s role as a pivotal treatment option for HER2-positive gastric cancer.
Key Findings
- Overall Survival Improvement: ENHERTU demonstrated a statistically significant improvement in OS, marking the first HER2-targeted treatment to show such a benefit in the second-line setting for metastatic gastric cancer.
- Positive Secondary Endpoints: In addition to OS, ENHERTU outperformed chemotherapy in key secondary endpoints, including PFS and ORR, underscoring its potential to slow disease progression and improve response rates.
- Consistent Safety Profile: The safety profile of ENHERTU in this trial was consistent with its established profile in previous studies, suggesting it can be used safely in clinical practice.
- Global Trial Data: The inclusion of diverse patient populations from Asia, Europe, and South America adds generalizability to the findings and suggests ENHERTU’s efficacy across different demographic groups.
Key Takeaway Messages
- ENHERTU’s Role in Second-Line Treatment: ENHERTU has shown the potential to be the first HER2-targeted therapy to offer survival benefits in second-line metastatic gastric cancer after progression on trastuzumab-based regimens, a previously unmet need in this patient population.
- Significance of the Findings: These results represent a breakthrough in the treatment of HER2-positive gastric cancer, offering hope for patients who previously had limited treatment options after first-line failure.
- Regulatory Implications: The positive interim results from the DESTINY-Gastric04 trial will likely lead to expanded indications for ENHERTU in global markets, including potential regulatory approvals for second-line treatment in regions where it is not currently approved.
- Safety Considerations: The favorable safety profile of ENHERTU, consistent with prior studies, is an important consideration for its broader clinical use, ensuring that its benefits outweigh potential risks in this patient population.
Written by Sona Karamyan, MD


