Chordoma is a rare, slow-growing malignant tumor that arises from remnants of the notochord, a structure present during early fetal development. These tumors typically occur along the spine, with the most common locations being the base of the skull (clivus) and the sacrum. Despite their slow growth, these tumors are locally invasive and can cause significant destruction to surrounding tissues, often leading to neurological symptoms.
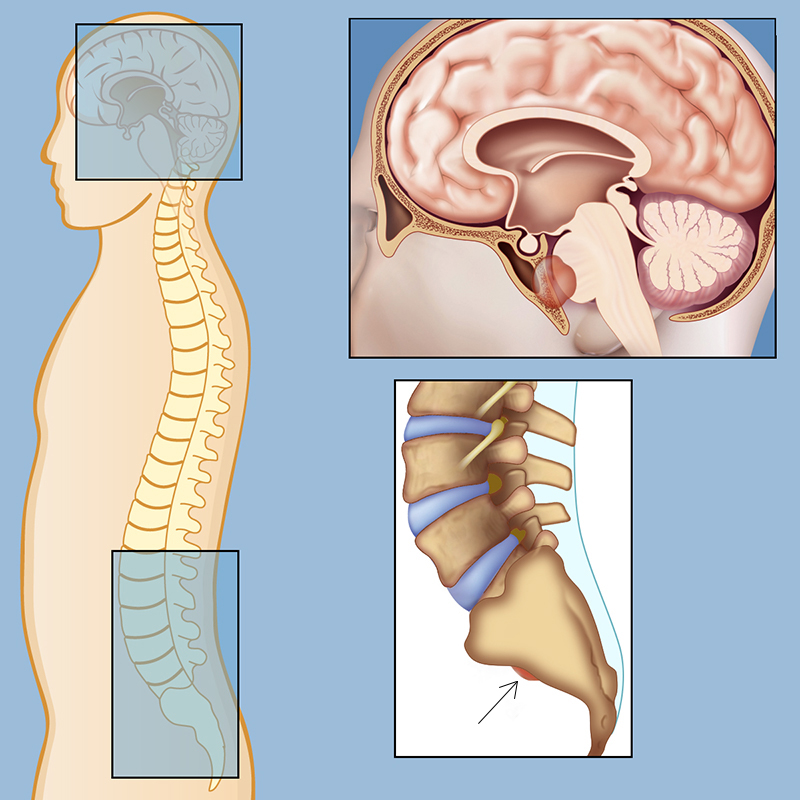
Chordomas in the spinal column and the skull base. The image is taken from neurosurgery.weillcornell.org.
Prevalence and Epidemiology
Chordomas are extremely rare, with an incidence of approximately 0.08 to 0.88 per 100,000 persons per year, varying between countries and races. In the United States, the annual incidence is about 350 cases, translating to roughly 1 per million people. These tumors account for 1% to 4% of all primary bone malignancies and about 20% of primary spinal tumors. They are more common in men, with a male-to-female ratio ranging from 1.5:1 to 2:1.
The most common anatomical locations for these tumors are the sacrum (50%), skull base (35%), and mobile spine (10-15%). The incidence peaks in individuals aged 40 to 70 years, although they can occur at any age, including in children. Pediatric chordomas are rare, accounting for less than 5% of cases, and tend to present with larger tumors and more aggressive behavior.
Types of Chordoma
Chordomas are classified into several histological subtypes, each with distinct characteristics and prognostic implications.
- Conventional Chordoma: This subtype account for approximately 95% of all chordoma cases. They have a relatively better prognosis compared to other subtypes, but they still exhibit high recurrence rates due to their locally invasive nature.
- Chondroid Chordoma: This subtype is most commonly found at the base of the skull. They generally have a better prognosis than conventional chordomas, with lower recurrence rates.
- Dedifferentiated Chordoma: This rare and aggressive subtype characterized by rapid growth and a high propensity for metastasis, dedifferentiated chordomas have the worst prognosis among all subtypes, with significantly lower overall survival rates.
- Poorly Differentiated Chordoma: This subtype is more common in pediatric and young adult patients. They have a distinct clinical profile and a poorer prognosis compared to conventional and chondroid chordomas and require aggressive multimodal treatment.
Causes and Risk Factors
Causes
- Notochordal Remnants: These tumors develop from leftover cells of the notochord, which is a flexible rod-like structure that provides support to the developing embryo. During fetal development, the notochord is typically replaced by the vertebral column, but in some cases, remnants of these cells persist and can give rise to chordomas later in life.
- Genetic Factors
Brachyury Gene (TBXT): A significant number of chordoma cases are associated with a mutation in the brachyury gene (crucial in embryonic development as it regulates the expression of genes involved in the formation of the mesoderm and notochord during gastrulation). This genetic variation increases the risk of developing chordoma, although it does not directly cause the disease.
Familial Chordoma: In rare instances, chordoma can occur in multiple members of the same family, suggesting a hereditary component. Some familial cases have been linked to duplications of the brachyury gene.
Tuberous Sclerosis Complex (TSC): There is an increased incidence of chordoma in children with Tuberous Sclerosis Complex, a genetic disorder caused by mutations in the TSC1 or TSC2 genes (regulate cell growth). This condition predisposes individuals to various tumors, including chordoma.
Risk Factors
- Age and Gender: These tumors are more commonly diagnosed in adults between the ages of 40 and 70, with a slight predominance in males.
- No Known Environmental, Dietary, or Lifestyle Risk Factors: There are no established environmental, dietary, or lifestyle factors that have been linked to an increased risk of developing this tumor. The majority of cases appear to occur sporadically without any identifiable external cause.
- Genetic Predisposition: As mentioned, genetic factors such as the presence of the brachyury gene SNP and familial history of chordoma can increase the risk, although these cases are rare
Symptoms
The symptoms depend on the tumor’s location and size. Common symptoms include:
Skull Base Chordoma
- Double Vision: The most common presenting symptom.
- Headache: Due to pressure on surrounding structures.
- Facial Numbness or Tingling: Resulting from nerve compression.
- Loss of Vision: If the optic nerves are affected.
- Hearing Loss: Due to involvement of auditory pathways.
- Difficulty Swallowing: If the tumor compresses the esophagus.
- Pituitary Gland Dysfunction: Leading to hormonal imbalances.
Spinal Chordoma
- Back Pain: The most common symptom, often severe and persistent.
- Numbness or Weakness: In the arms or legs, depending on the tumor’s location.
- Loss of Bladder or Bowel Control: If the sacral nerves are involved.
- Sexual Dysfunction: Due to nerve damage.
Diagnosis
Diagnosing typically involves a combination of imaging studies and biopsy:
- Magnetic Resonance Imaging (MRI): The most useful test for visualizing the extent of the tumor and its relationship to surrounding structures.
- Computed Tomography (CT) Scan: Often used to assess bone involvement and rule out metastasis.
- Biopsy: A needle biopsy is usually performed to obtain a tissue sample for histological confirmation.
Treatment Options for Chordoma
Surgery
Surgery is the cornerstone of chordoma treatment and aims to achieve complete tumor resection with clear margins. The extent of surgical resection is one of the most critical prognostic factors for chordoma patients. Types of surgical approaches include:
- En Bloc Resection: This involves removing the tumor in one piece along with a margin of healthy tissue. It is considered the gold standard for chordomas of the mobile spine and sacrum due to its association with lower recurrence rates and longer survival.
- Endoscopic Endonasal Approach (EEA): For skull base chordomas, this minimally invasive technique allows surgeons to access the tumor through the nasal passages, avoiding external incisions. EEA offers benefits such as no disfigurement and faster recovery times.
- Microsurgery: Utilizes high-powered microscopes to distinguish the tumor from surrounding healthy tissues, minimizing the risk of nerve damage during surgery.
Radiation Therapy
Radiation therapy is typically used as an adjunct to surgery, especially when complete resection is not possible. Advances in radiation technology have improved the efficacy and safety of this treatment modality. Studies have shown that adjuvant radiation therapy improves overall survival in patients with positive surgical margins. In cases where surgery is not feasible, radiation therapy alone can provide reasonable disease control, although it is generally less effective than combined modality treatment.
- Proton Beam Therapy (PBT): Preferred for chordomas due to its ability to deliver high doses of radiation precisely to the tumor while sparing surrounding healthy tissue. PBT is particularly effective for tumors located near critical structures like the brainstem and spinal cord.
- Carbon Ion Radiotherapy (C-ion RT): Offers better relative biological effectiveness compared to protons and photons, making it a promising option for chordomas. C-ion RT is available in select centers worldwide and is associated with high local control rates.
- Stereotactic Radiosurgery (SRS): Uses multiple beams of radiation to deliver a high dose to a small, targeted area. SRS is often used for recurrent or inoperable chordomas.
- Intensity-Modulated Radiation Therapy (IMRT): A type of photon radiation that allows for precise targeting of the tumor, minimizing damage to surrounding tissues.
Chemotherapy
These tumors are generally resistant to conventional chemotherapy, but certain drugs and combinations have shown promise in clinical trials.
Targeted Therapy and Immunotherapy
Recent advances in molecular biology have led to the development of targeted therapies and immunotherapies for this tumor
Targeted Therapies
- EGFR Inhibitors: These drugs target the epidermal growth factor receptor (EGFR)* and have shown efficacy in clinical trials for advanced or metastatic chordoma.
- Brachyury Inhibitors: Brachyury is a transcription factor overexpressed in chordomas. Targeting this protein is a promising area of research.
- Other Targets: Drugs targeting PDGFR, VEGFR*, and other molecular pathways involved in chordoma pathogenesis are under investigation.
*These proteins are growth promoting receptors, activation of which may lead to constant cellular division and development of tumors.
Immunotherapy
- Checkpoint Inhibitors: Drugs which target immune checkpoints, are being tested in clinical trials for their ability to enhance the immune response against chordoma cells.
- Cancer Vaccines: Experimental vaccines aim to stimulate the immune system to recognize and attack chordoma cells. These are still in the early stages of research.
More information about completed and ongoing clinical trials for this tumor can be found here – clinicaltrials.gov.
What information should chordoma patients have regarding surgical options? The video is created by the Chordoma Foundation.
Prognosis
The prognosis for chordoma patients varies significantly based on several factors, including the tumor’s location, size, histological subtype, and the extent of surgical resection. The overall 5-year survival rate (the survival rate is the percentage of people who are still alive after a certain period of time following a diagnosis or treatment for a disease) is approximately 50%. Sacral chordomas tend to have the worst prognosis, while skull base chordomas have slightly better outcomes.
Factors associated with a poorer prognosis include older age, female sex, larger tumor size, incomplete surgical resection, presence of metastasis, local recurrence, and dedifferentiated histological subtype. Complete surgical resection combined with high-dose radiation therapy offers the best chance for long-term survival.
Patient Survivorship
Survivorship in chordoma patients encompasses the period from diagnosis through the remainder of life, including the time during and after treatment. The Chordoma Foundation emphasizes that survivorship care focuses on the overall health and well-being of a person who has been through treatment for cancer. This care includes addressing physical, emotional, spiritual, and practical issues that a cancer survivor and their loved ones may face beyond diagnosis and treatment.
Key Aspects of Survivorship Care
- Physical Health: Regular follow-up appointments are crucial to monitor for recurrence and manage any ongoing side effects of treatment. This includes imaging studies like MRIs and CT scans, as well as blood tests.
- Emotional and Mental Health: Psychological support is essential, as the journey through cancer treatment can be emotionally taxing. Support groups, counseling, and mental health services can help manage stress, anxiety, and depression.
- Practical Issues: Survivorship care also involves practical aspects such as managing work responsibilities, understanding workplace rights, and making necessary lifestyle adjustments to accommodate any physical limitations.
Problems During and After Treatment
Chordoma treatment, which often involves surgery and radiation therapy, can lead to a range of complications and side effects. These can be immediate or develop over time.
During Treatment
- Surgical Complications: Common complications include wound infections, dehiscence, and sacral fractures. Intraoperative complications can also include significant blood loss and damage to surrounding tissues.
- Radiation Therapy Side Effects: Acute side effects of radiation therapy can include dermatitis, pain, nausea, vomiting, mucositis, and diarrhea. Long-term side effects may include neuropathy, soft tissue necrosis, and secondary malignancies.
After Treatment
- Pain Management: Pain is a common issue for chordoma patients, both during and after treatment. Effective pain management strategies include medications, physical therapy, and, in some cases, nerve blocks.
- Neurological Complications: Depending on the tumor’s location, patients may experience neurological deficits such as weakness, numbness, or loss of bladder/bowel control. Rehabilitation therapies can help manage these symptoms.
- Functional Impairments: Long-term functional impairments may include difficulties with mobility, bladder/bowel control, and sexual function. Rehabilitation and supportive therapies are crucial for improving quality of life.
- Psychological Support: The diagnosis and treatment of this tumor can be emotionally challenging. Psychological support and counseling can help patients and their families cope with the stress and anxiety associated with the disease.
Recommendations for Patients
- Regular Follow-Up: Consistent follow-up care is essential to monitor for recurrence and manage any ongoing side effects. This includes regular imaging studies and consultations with the healthcare team.
- Pain Management: Work with the healthcare team to develop a comprehensive pain management plan. This may include medications, physical therapy, and other interventions to manage pain effectively.
- Rehabilitation: Engage in rehabilitation therapies to address any functional impairments resulting from treatment. This can include physical therapy, occupational therapy, and speech therapy, depending on your specific needs.
- Psychological Support: Seek psychological support to manage the emotional and mental health challenges associated with chordoma. Support groups, counseling, and mental health services can be beneficial.
- Lifestyle Adjustments: Make necessary lifestyle adjustments to accommodate any physical limitations. This may include modifying your living environment, adjusting your work responsibilities, and adopting a healthy lifestyle to support your overall well-being.
- Stay Informed: Keep informed about the condition and treatment options. Organizations like the Chordoma Foundation offer valuable resources and support.
The video is created by the Chordoma Foundation.
Conclusion
Chordoma is a rare and challenging malignancy, but advancements in treatment and comprehensive survivorship care offer hope for improved outcomes and quality of life. Multidisciplinary approaches involving surgery, radiation therapy, and emerging targeted therapies are enhancing patient prognosis. Survivorship care plans, addressing both physical and emotional needs, are crucial for managing the long-term effects of treatment. With ongoing research and support from various organizations, patients and their families can navigate this journey with greater confidence and optimism. By staying informed, seeking support, and engaging in regular follow-up care, chordoma patients can look forward to a future with better treatment options and improved survivorship.
Sources
- National Cancer Institute – cancer.gov
- American Cancer Society – cancer.org
- National Organization for Rare Disorders – rarediseases.org
- Chordoma Foundation
- American Society of Clinical Oncology – Cancer.net
- Chordoma—Current Understanding and Modern Treatment Paradigms – Journal of Clinical Medicine
- Surgical management of chordoma: A systematic review – The Journal of Spinal Cord Medicine


