
FORTITUDE-101 Study Updates 2025: Bemarituzumab Plus mFOLFOX6 in FGFR2b-Positive Gastric and GEJ Cancer
On June 30, 2025, Amgen announced positive topline results from the Phase 3 FORTITUDE-101 study evaluating bemarituzumab plus chemotherapy (mFOLFOX6) as first-line treatment in patients with unresectable, locally advanced or metastatic gastric or gastroesophageal junction (G/GEJ) cancer with FGFR2b overexpression. The study met its primary endpoint of overall survival (OS) at a pre-specified interim analysis, representing a major advancement in the targeted treatment of this aggressive cancer subtype.
FORTITUDE-101 Study Design
FORTITUDE-101 is a randomized, double-blind, placebo-controlled Phase 3 trial designed to compare bemarituzumab plus mFOLFOX6 to placebo plus mFOLFOX6 in the first-line treatment of advanced G/GEJ cancer. Eligibility required FGFR2b protein overexpression, defined as 2+/3+ staining in ≥10% of tumor cells by centralized immunohistochemistry (IHC) testing. Patients who were HER2-positive were excluded.
The study enrolled 547 patients across 300 sites in 37 countries. FGFR2b overexpression prevalence in ≥10% of tumor cells occurred in approximately 16% of screened patients, aligning with prior epidemiologic estimates.
Primary and Secondary Endpoints
The primary endpoint is overall survival (OS) in the FGFR2b ≥ 10 % population. Key secondary endpoints include progression-free survival (PFS), overall response rate (ORR), duration of response, disease-control rate and safety.
FORTITUDE-101 Study Results
At the interim analysis, bemarituzumab plus chemotherapy showed a statistically significant and clinically meaningful improvement in OS compared to chemotherapy alone. While detailed OS and PFS statistics have not yet been publicly released, the magnitude of benefit was sufficient to meet the pre-specified criteria for success.
Amgen emphasized the high unmet need in gastric cancer, the fifth leading cause of cancer-related deaths globally, with most patients diagnosed at an advanced stage. These data offer the first positive Phase 3 results for an FGFR2b-targeted monoclonal antibody in this setting.
A key component of FORTITUDE-101 was enhanced ocular safety monitoring, due to previously observed eye toxicities in earlier trials. The most frequent treatment-emergent adverse events (>25%) in the bemarituzumab arm included:
- Reduced visual acuity
- Punctate keratitis
- Corneal epithelium defects
- Dry eye
- Nausea
- Neutropenia
- Anemia
These events were generally consistent with the Phase 2 experience but occurred with greater frequency and severityin the Phase 3 bemarituzumab arm. Detailed efficacy and safety data are expected to be presented at an upcoming medical congress. A separate Phase 3 trial of bemarituzumab in combination with nivolumab for FGFR2b-positive gastric cancer is ongoing, with results anticipated in H2 2025.
More information is available in AMGEN Official Website.
What is Bemarituzumab and How Does it Work?
Bemarituzumab is a first-in-class, humanized monoclonal antibody that selectively targets the fibroblast growth factor receptor 2 isoform IIIb (FGFR2b), which is overexpressed on the surface of certain gastric cancer cells. By binding to FGFR2b, bemarituzumab blocks the receptor’s activation and downstream signaling pathways that promote tumor cell growth, survival, and migration.
In addition to receptor blockade, bemarituzumab engages the immune system through antibody-dependent cellular cytotoxicity (ADCC), recruiting immune cells to kill FGFR2b-expressing tumor cells. This dual action—both direct inhibition of cancer cell signaling and immune-mediated tumor cell destruction—makes bemarituzumab a promising targeted therapy for patients with FGFR2b-positive advanced gastric and gastroesophageal junction cancers.
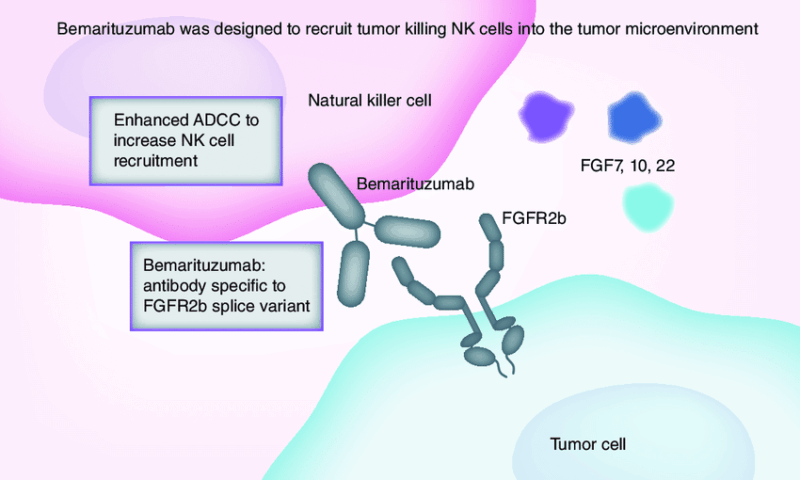
Source: ResearchGate
About FGFR2b
FGFR2b (fibroblast growth factor receptor 2b) is a splice variant of the FGFR2 gene. Overexpression of FGFR2b promotes abnormal tumor signaling and cell proliferation. IHC analysis shows that FGFR2b is overexpressed in approximately 38% of advanced G/GEJ cancers, with 16% of tumors exhibiting ≥10% of cells with 2+/3+ intensity. This makes FGFR2b an emerging biomarker and potential therapeutic target in gastric cancer.

FGFR2b Prevalence Study Published in JCO Precision Oncology
Earlier in January 2025, a pivotal study evaluating FGFR2b protein overexpression in advanced gastric and gastroesophageal junction (G/GEJ) cancers was published in JCO Precision Oncology. The article, titled “Prevalence of FGFR2b Protein Overexpression in Advanced Gastric Cancers During Prescreening for the Phase III FORTITUDE-101 Trial,” provided key insights into the biomarker landscape that underpins the FORTITUDE-101 trial.
Study Objectives and Methods
The aim of the JCO study was to assess the global prevalence of FGFR2b protein overexpression using a validated immunohistochemistry (IHC) assay in tumor samples from patients with newly diagnosed advanced or metastatic G/GEJ cancer. Over 3,700 samples from 37 countries were centrally analyzed using the VENTANA FGFR2b (FPR2-D) RxDx Assay.
Two levels of FGFR2b expression were reported:
- Any % 2+/3+ Tumor Cells (TC): FGFR2b positivity in 37.8% of evaluable samples
- ≥10% 2+/3+ TC (Trial Eligibility Threshold): FGFR2b positivity in 16.2% of samples
These findings confirmed that a significant subset of patients with G/GEJ cancer harbor FGFR2b overexpression, particularly at the ≥10% threshold required for eligibility in FORTITUDE-101.

Key Findings and Implications
Importantly, the study concluded that FGFR2b overexpression was consistent across age, sex, biopsy method, tumor site, and geographic regions. This uniformity reinforces the global applicability of FGFR2b as a predictive biomarker.
The prevalence data from the JCO publication provided the foundational rationale for FGFR2b-targeted therapy with bemarituzumab and supported the trial design of FORTITUDE-101. In particular, patients whose tumors exhibited ≥10% 2+/3+ FGFR2b expression were identified as the subgroup most likely to benefit from targeted intervention—an assumption now validated by the positive Phase 3 results announced by Amgen.
Conclusion
The FORTITUDE-101 trial marks a pivotal step forward in the treatment of FGFR2b-positive gastric cancer. With significant OS improvement, bemarituzumab in combination with chemotherapy may represent a new targeted standard of care for this molecular subset. Full data disclosure at upcoming oncology meetings will further shape the future of this promising therapeutic approach.
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
