On April 23, 2025, the U.S. Food and Drug Administration (FDA) granted accelerated approval to penpulimab (AK105), a PD-1–blocking monoclonal antibody, for the treatment of adult patients with recurrent or metastatic (R/M) non-keratinizing nasopharyngeal carcinoma (NPC) who have experienced disease progression on or after platinum-based chemotherapy.
Overview of nasopharyngeal cancer
Nasopharyngeal carcinoma is a malignant tumor that arises from the epithelial lining of the nasopharynx, the area located behind the nose and above the back of the throat. It is distinct from other head and neck cancers due to its unique geographic distribution, risk factors, and histopathology. The disease is rare globally but is highly prevalent in Southern China, Southeast Asia, North Africa, and some Arctic regions. It most commonly affects individuals between the ages of 40 and 60 and is more frequent in males.
Source: www.oncodaily.com
The strongest risk factor for nasopharyngeal carcinoma is infection with the Epstein-Barr virus, especially in the non-keratinizing and undifferentiated histological subtypes. Other contributing factors include genetic susceptibility, consumption of preserved foods such as salted fish that contain nitrosamines, exposure to wood dust and formaldehyde, and a family history of the disease.
The World Health Organization classifies nasopharyngeal carcinoma into keratinizing squamous cell carcinoma, non-keratinizing differentiated carcinoma, and non-keratinizing undifferentiated carcinoma, the last of which is the most common and closely linked to EBV.
Symptoms often arise from local tumor growth or regional lymph node metastasis. These may include a painless neck mass, nasal congestion or bleeding, hearing loss, tinnitus, ear infections, and in advanced cases, cranial nerve deficits due to skull base invasion.
Diagnosis is typically confirmed through nasopharyngoscopy with biopsy, supported by imaging studies such as MRI of the head and neck, chest CT, and PET-CT for staging. Plasma Epstein-Barr virus DNA levels are often used as a biomarker for diagnosis, prognosis, and monitoring.
The mainstay of treatment is radiotherapy, especially intensity-modulated radiotherapy, often combined with concurrent chemotherapy for locally advanced disease. Neoadjuvant chemotherapy may be used in high-risk cases. Immunotherapy, particularly immune checkpoint inhibitors like anti–PD-1 agents, is emerging as a promising option in recurrent or metastatic settings. Surgery plays a limited role, mainly in salvage situations for persistent or recurrent disease
What drug is Penpulimab and how does it work?
Penpulimab (AK105) is a fully human IgG1 anti–PD-1 monoclonal antibody co-developed by Akeso, Inc. and Chia Tai Tianqing Pharmaceutical Group Co., Ltd. (Sino Biopharm). It is designed for the treatment of multiple cancers, with a particular focus on improving safety and efficacy compared to earlier PD-1/PD-L1 inhibitors.
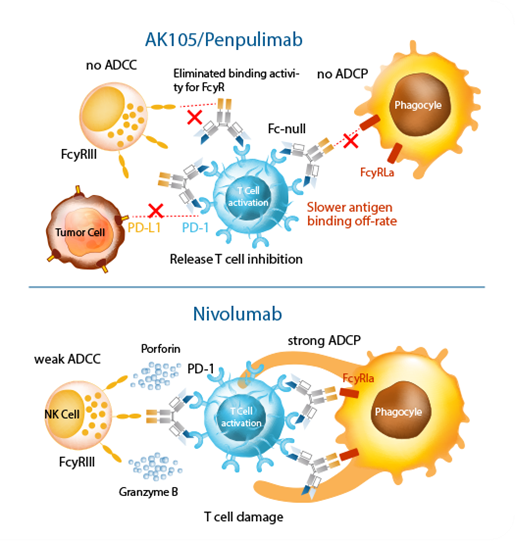
- Penpulimab is the first approved PD-1 antibody with an IgG1 subtype and Fc modification to completely eliminate Fcγ receptor binding. This helps:
- Reduce antibody-dependent cellular phagocytosis (ADCP) of T cells
- Minimize immune-related adverse events (irAEs)
- Enhance T-cell-mediated anti-tumor immunity
The Fc-engineered design aims to avoid overstimulation of the immune system, reducing the risk of severe irAEs typically observed with standard PD-1 inhibitors.
Source: www.akesobio.com
Approved Indications
As of 2025, Penpulimab has received approval in China and the U.S. for:
- Relapsed or refractory classical Hodgkin lymphoma
- Recurrent or metastatic non-keratinizing nasopharyngeal carcinoma (FDA-approved April 2025)
Source: www.akesobio.com
Clinical Development
Penpulimab is being evaluated in multiple ongoing clinical trials targeting:
- Non-small cell lung cancer (NSCLC)
- Liver cancer
- Esophageal squamous cell carcinoma
- Head and neck squamous cell carcinoma
- Melanoma
Penpulimab offers a distinct strategic advantage through its high specificity and strong binding affinity to the PD-1 receptor, enabling effective blockade of the PD-1/PD-L1 pathway. Unlike conventional anti–PD-1 agents such as nivolumab and pembrolizumab, penpulimab features a unique Fc-null IgG1 backbone design, which eliminates Fcγ receptor binding. This innovation reduces the risk of immune-related adverse events by preventing antibody-dependent cellular phagocytosis (ADCP) of activated T cells. Additionally, penpulimab is a key component of Akeso’s broader immuno-oncology platform, which includes combination strategies with novel bispecific antibodies like cadonilimab to enhance therapeutic outcomes across various solid tumors.
Clinical Trial Basis
The approval was based on a single-arm, multicenter clinical trial involving 174 patients with R/M non-keratinizing NPC who had progressed after platinum-based therapy.
Treatment Regimen: Penpulimab 200 mg IV every 3 weeks until disease progression or unacceptable toxicity.
Efficacy Results:
- Objective Response Rate (ORR): 26.4% (95% CI: 19.9%, 33.8%)
- Median Duration of Response (DoR): 12.4 months
- Complete Response (CR): 3.4%
- Partial Response (PR): 23.0%
Safety Profile
Most common adverse reactions (≥10%): rash, hypothyroidism, pruritus, fatigue, decreased appetite, nausea, diarrhea, and cough.
Immune-mediated adverse events were consistent with other PD-1 inhibitors.
How does this approval change the treatment landscape?
This drug Introduces a New Immunotherapy Option. This is the first FDA-approved anti–PD-1 therapy specifically indicated for non-keratinizing NPC after progression on platinum-based chemotherapy. Prior to this, patients had limited options and were often treated off-label with other PD-1/PD-L1 inhibitors based on limited data.
Penpulomab Offers a Safer PD-1 Inhibitor Alternative. Penpulimab’s Fc-engineered IgG1 backbone eliminates Fcγ receptor binding, which may reduce immune-related adverse events compared to conventional PD-1 inhibitors like nivolumab and pembrolizumab. This makes it particularly valuable for patients who may be at higher risk of toxicity.
NPC, especially the non-keratinizing subtype, is closely associated with Epstein-Barr virus (EBV) and a highly inflamed tumor microenvironment. This approval reinforces the role of immune checkpoint blockade in virus-associated cancers and supports continued development of immunotherapy in EBV-driven malignancies.
You can read the full article here
Written by Sona Karamyan, MD