Biagio Ricciuti, Medical Oncologist at Dana-Farber Cancer Institute, shared on X/Twitter:
”Our work on long-term outcomes to PD-1 monotherapy by increasing PD-L1 levels in NSCLC is out in JTO and JTO CRR.
PD-L1 TPS ≥90% (vs 50-89%) is associated with higher 3-year PFS and OS rates and favorable genomic and immunophenotypic profiles.
Responses to first-line PD-1 inhibition vary among patients with metastatic NSCLC and a PD-L1 TPS ≥50%. We previously reported improved outcomes to first-line pembrolziumab in patients with NSCLC with a PD-L1 TPS of ≥90% vs 50-89% in a pilot study.
Here, we report the 3-year survival with first-line pembrolizumab and cemiplimab in two large independent cohorts of patients with PD-L1 TPS ≥90% vs 50-89% and characterize genomic and immunophenotypic differences between these PD-L1 groups, which were largely unknown.
We first analyzed clinical outcomes to commercial pembrolizumab (PD-1 inhibitor) by increasing PD-L1 TPS levels in a retrospective cohort. We noted significantly higher ORR and 3 year PFS (30% vs 14%) and OS (46% vs 31%) rates among pts with PD-L1 TPS ≥90% vs 50-89%.
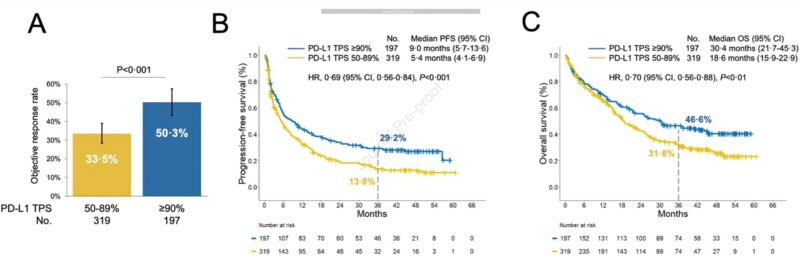
To validate these findings we also examined outcomes to cemiplimab (PD-1 inhibitor) in the randomized phase III trial EMPOWER Lung 01, and confirmed significantly improved 3 year outcomes among patients with PD-L1 TPS ≥90% vs 50-89%.
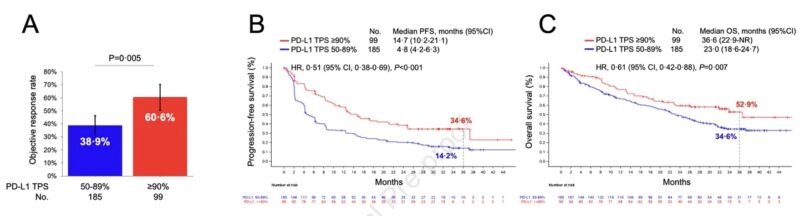
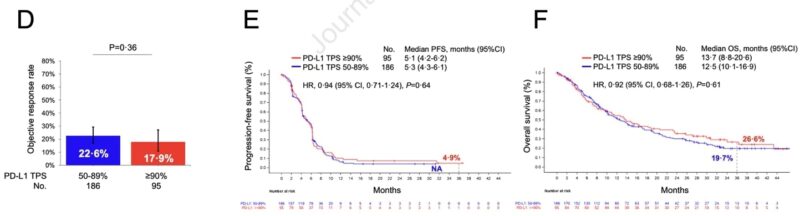
Importantly, there was no difference in ORR, PFS, and OS according to PD-L1 expression (≥90% vs 50-89%) in patients who were randomized to platinum-based chemotherapy in the EMPOWER Lung 01 trial.
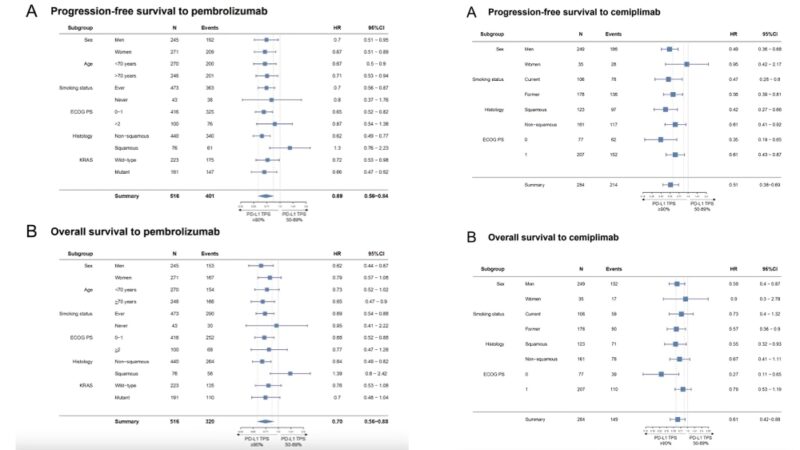
Subgroup analyses confirmed improved PFS and OS in patients with NSCLC and PD-L1 TPS ≥90% vs 50-89% in most groups. Main exceptions were patients with squamous histology and those who never smoked in the pembrolizumab cohort.
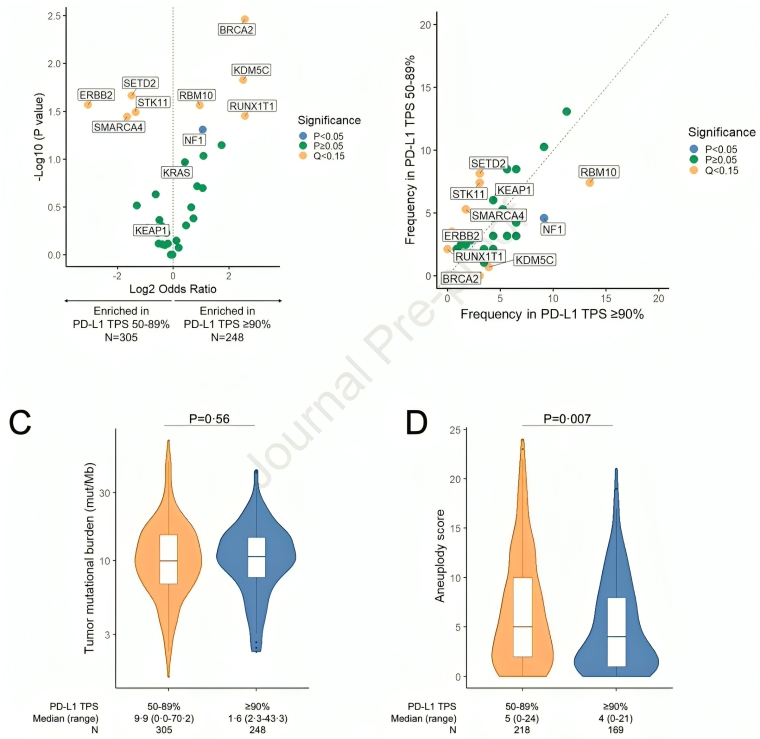
We next asked if NSCLCs with PD-L1 ≥90% and 50-89% had different genomic profiles. We found that NSCLCs with PD-L1 TPS of 50-89% were enriched in SMARCA4 and STK11 mutations, and had higher aneuploidy levels. BRCA2 and KDM5C mutations were enriched in NSCLCs with PD-L1 ≥90%.
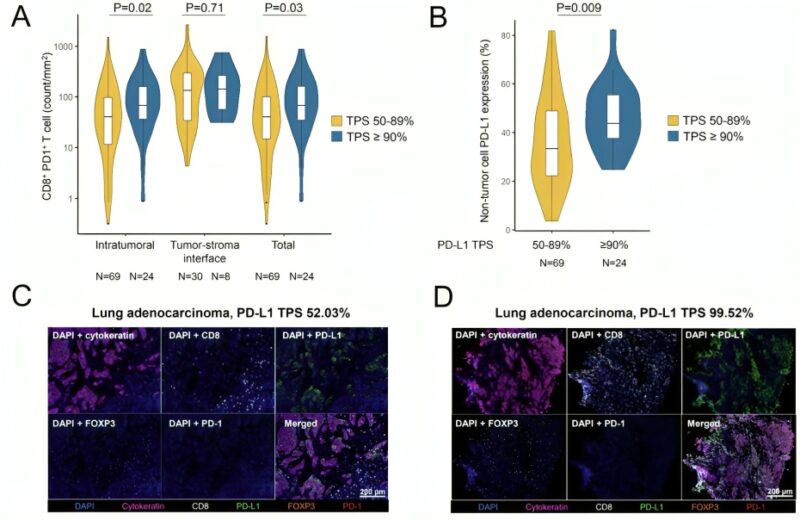
We lastly examined the immunophenotype of these NSCLCs, and noted a significantly higher density of intratumoral CD8+ PD1+ T cells as well higher PD-L1 expression on non-tumor cells in NSCLCs with a PD-L1 TPS ≥90% vs 50-89%.
Our findings indicate that NSCLCs with a PD-L1 ≥90% have distinct genomic and immunophenotypic profiles, and improved long-term outcomes with PD-1 monotherapy. These results can have implication for clinical decision making as well as for trial design/interpretation.
This study was possible thanks to our patients and their families, the Regeneron Lung team and wonderful collaborators at Memorial Sloan Kettering Cancer Center, MD Anderson Cancer Center, MGH Thoracic Oncology and Dana-Farber Cancer Institute.”
Source: Biagio Ricciuti/X


