
Paolo Tarantino: Top 10 ADC Milestones of 2024
Paolo Tarantino, Clinical Research Fellow at the Dana-Farber Cancer Institute, shared on X:
“2024 has been a remarkable year for ADCs. We saw the first agnostic ADC approval, the first subq ADC, 77 new ADCs in the clinic, multi-billion dollar deals sealed and so much more.
I sat with Raffaele Colombo, to review this terrific year.
Here are our Top 10 ADC Milestones of 2024:
1. T-DXd AGNOSTIC APPROVAL.
T-DXd was approved by the FDA to treat patients with advanced HER2-positive (IHC 3+) solid tumors, becoming the first ADC (and first anti-HER2 treatment) to ever gain a tumor-agnostic regulatory approval.
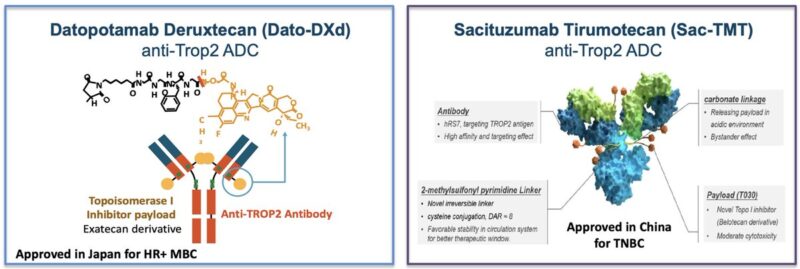
2. TWO NEW TROP2 ADCs IN THE WORLD.
The Trop2 targeted ADCs Sacituzumab Tirumotecan (Sac-TMT) and Datopotamab deruxtecan (Dato-DXd) were approved to treat MBC in China and Japan, respectively.
Both drugs may also become available in western countries in the coming months/years.
3. EV+pembro and MIRV approved in European Union.
After being approved by the FDA in Dec 2023, the 1L mUC combo of enfortumab vedotin + pembro was approved by the EMA in 2024. Similarly, mirvetuximab soravtansine was recently approved in Europe for patients with advanced ovarian cancer.

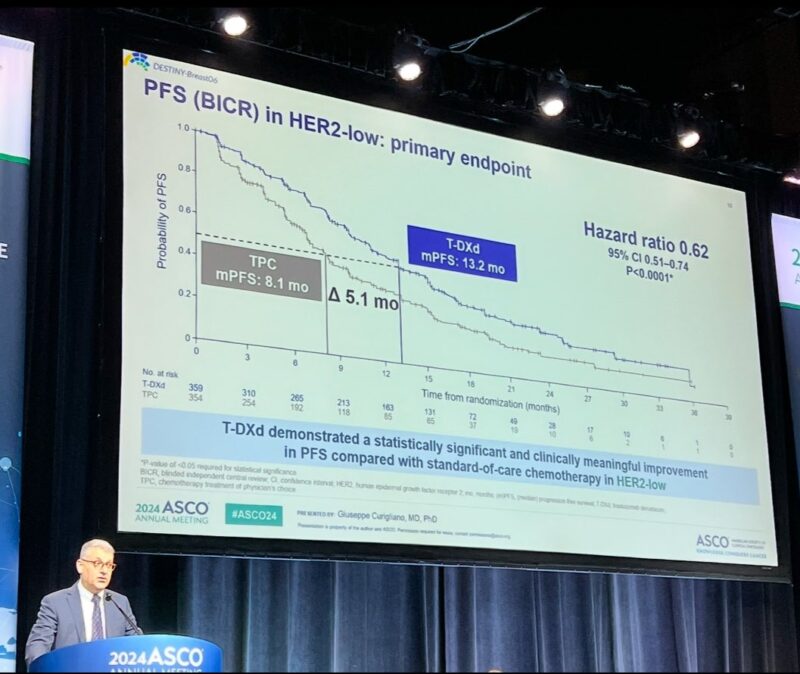
4. DESTINY-Breast06.
T-DXd outperformed 1L chemotherapy for the most common subtype of MBC (HR+/HER2-), and showed impressive activity even in HER2-0 (ultralow) MBC, challenging the canonical boundaries of HER2 targetability.
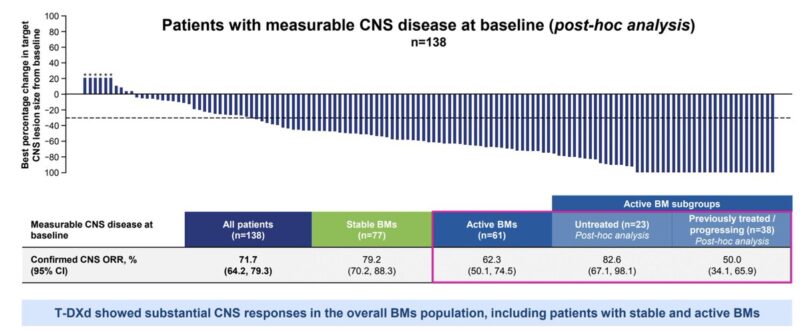
5. DESTINY-Breast12.
T-DXd exhibited outstanding intracranial activity among patients with HER2+ breast cancer brain metastases, including those with active mets. Based on this data, T-DXd is now considered the preferred 2L treatment in this population.
6. DUAL ADC TRIAL.
2024 saw the publication of DAD (Dual ADC), the first trial combining 2 different ADCs. Among patients with highly pretreated mUC, the combo of EV+SG achieved relevant activity (ORR 70%). Currently under testing with pembro (DAD-IO).

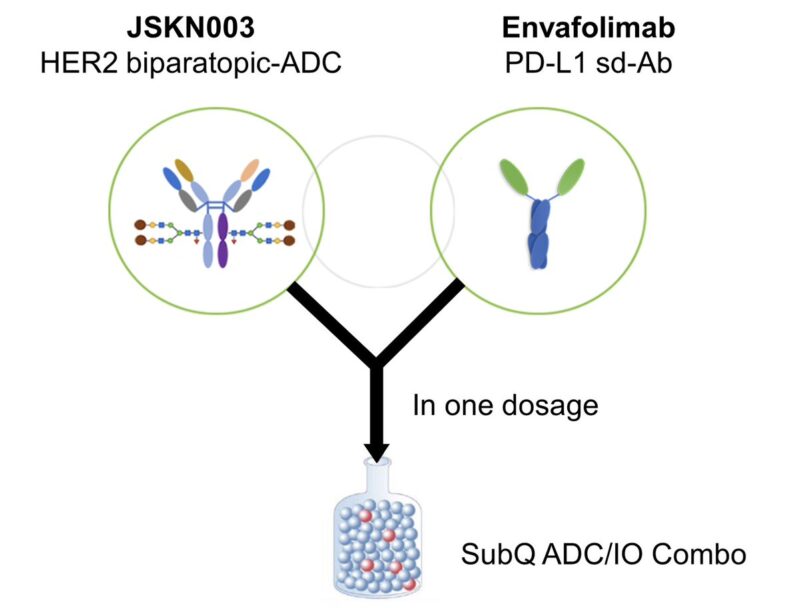
7. FIRST SUBCUTANEOUS ADC.
In 2024, we saw the first clinical report of the subcutaneous combination of JSKN003 (bispecific HER2 ADC) and envafolimab (anti-PDL1). The JSKN033-101 phase 1/2 study found the sc formulation to be well tolerated and active.
8. ADC IMPROVES OUTCOMES IN MULTIPLE MYELOMA.
In 2024, belantamab mafodotin combinations have been filed across the world for treating relapsed or refractory multiple myeloma based on the results of the DREAMM-7 and DREAMM-8 trials.
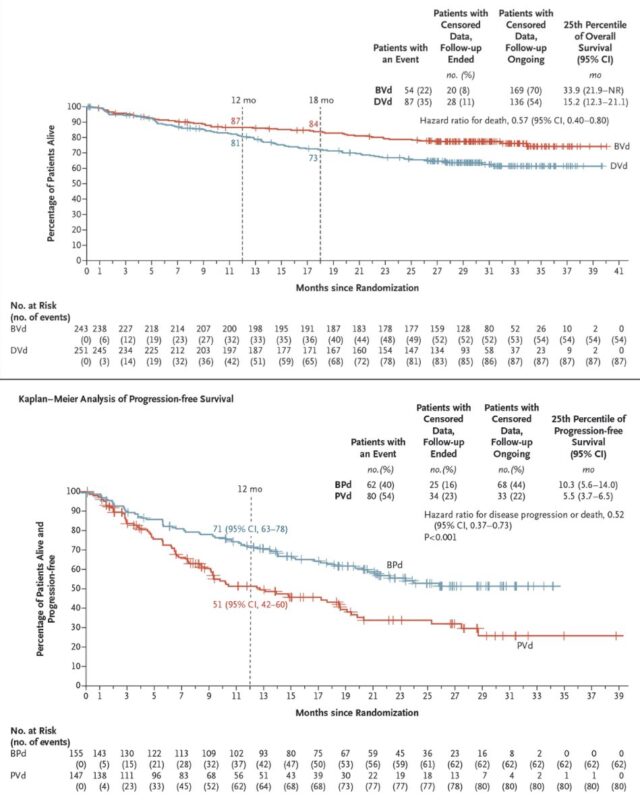
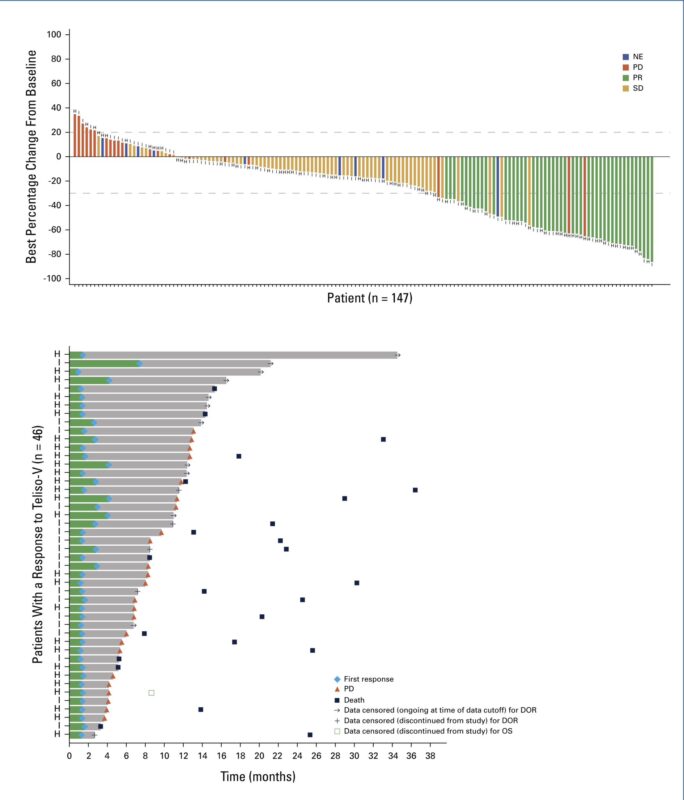
9. LUMINOSITY.
BLA submission for accelerated approval of telisotuzumab vedotin (Teliso-V), supported by data from the Phase 2 LUMINOSITY trial (ASCO24), where Teliso-T achieved ~30% ORR in advanced, c-Met+ NSCLC. Teliso-V may be approved in 2025.
10. ADC acquisition, licensing, partnership, and collaboration frenzy.
More than 30 deals sealed in 2024, including two large acquisitions: Johnson and Johnson acquried Ambrx Biopharma for $2.0 billion and Genmab acquired ProfoundBio for $1.8 billion.”

Dr. Paolo Tarantino, MD, is pursuing an advanced research fellowship at Dana-Farber Cancer Institute and Harvard Medical School, concurrently working towards a PhD in clinical research at the University of Milan.
His research focuses on exploring the HER2 oncoprotein, investigating the emerging HER2-low subgroup of breast tumors, and developing novel antibody-drug conjugates targeting every subtype of breast cancer.
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
