
Four new drugs approved by the FDA in October summarized by Samuel Hume
Samuel Hume, Scientist at the University of Oxford, shared a post on X:
“The FDA approved 4 new drugs in October:
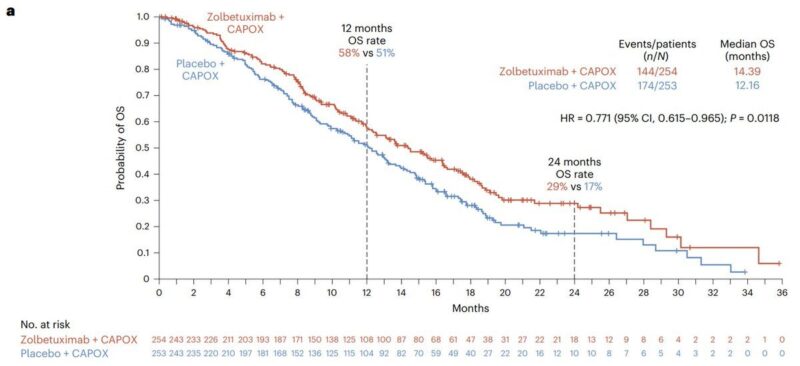
1. Zolbetuximab, for gastric cancer.
It’s a monoclonal antibody that targets the tight junction protein, CLDN18.2
CLDN18.2 is buried in tight junctions of normal stomach cells, but gets exposed in gastric cancer (~38% stain positive for CLDN18.2), accessible to cytolytic antibodies like Zolbetuximab
It was approved for CLDN18.2-positive, locally advanced unresectable or metastatic gastric or gastroesophageal junction adenocarcinoma
In two phase 3 trials, Zolbetuximab extended overall survival when combined with chemotherapy.
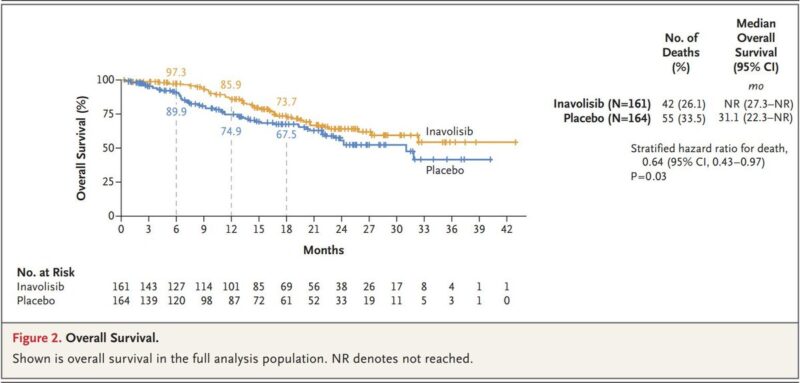
2. Inavolisib, for breast cancer.
Inavolisib inhibits the p110α subunit of PI3K, and promotes degradation of the mutant form – which makes it distinct from previous PI3K inhibitors, and widens its therapeutic window
It was approved for estrogen receptor-positive, HER2-negative, PI3KCA-mutated locally advanced or metastatic breast cancer
In this cancer type there’s 3 driving pathways: the estrogen receptor, CDK4/6, and PI3K
When Inavolisib was added to Palbociclib (a CDK4/6 inhibitor), and Fulvestrant (an estrogen receptor antagonist) it extended overall survival in a phase 3 trial.
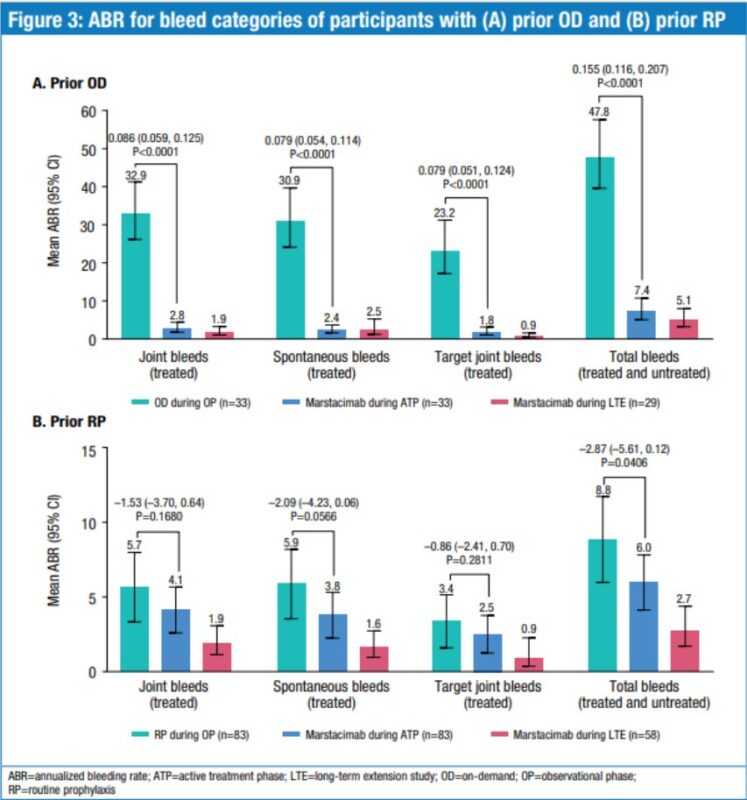
3. Marstacimab, for Haemophilia A/B.
It’s a monoclonal antibody that inhibits tissue factor pathway inhibitor, to boost clotting in a way that’s independent of factor VIII (mutant in Haemophilia A) and factor IX (mutant in Haemophilia B)
Haemophilia is typically managed with prophylactic or on-demand IV infusions of recombinant factor VIII or IX
Marstacimab is given subcutaneously (so could be self-administered by patients), and, in a phase 3 trial, it reduced bleeding rates vs. factor VIII or IX infusions.
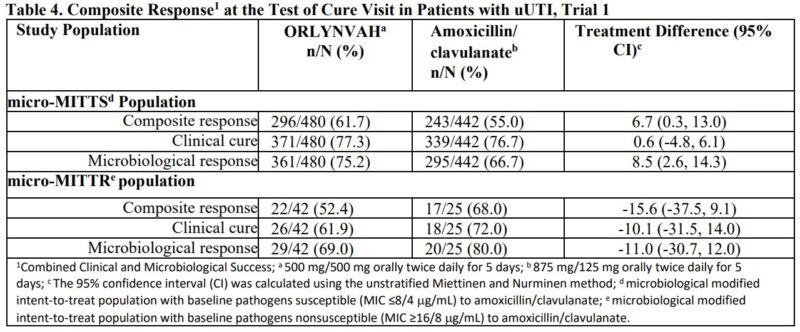
4. Sulopenem Etzadroxil-Probenecid, for antibiotic-resistant uncomplicated UTIs.
Sulopenem is a penem antibiotic, the Etzadroxil component allows it to be absorbed orally (unlike other penems, which are all given IV), and Probenecid is a renal transport inhibitor – to reduce renal excretion of the drug
It was approved to treat uncomplicated UTIs in women, caused by E. coli, K. pneumoniae, or P. mirabilis, that are resistant to other antibiotics
It was tested in two phase 3 trials (one vs. co-amoxiclav and the other vs. ciprofloxacin).”
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
