
Francisco Esteva: New treatment offering hope to those battling early breast cancer
Francisco Esteva, Chief of the Division of Hematology and Medical Oncology at Lenox Hill Hospital, shared a post on LinkedIn:
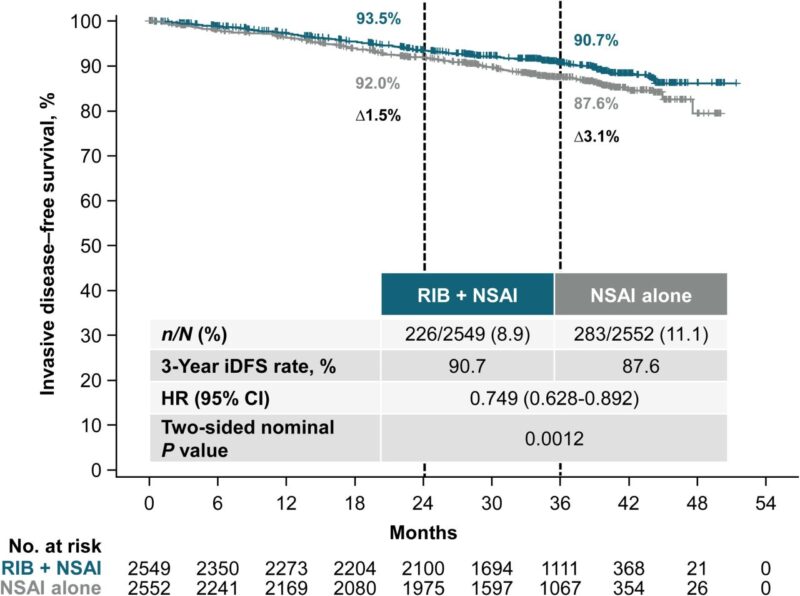
“A new treatment is offering hope to those battling early breast cancer: the NATALEE trial explored the addition of ribociclib – also known as Kisqali – to standard endocrine therapy for hormone receptor-positive, HER2-negative early breast cancer.
The trial involved over 5,000 participants, including both premenopausal and postmenopausal women and men. They were randomized to receive either ribociclib with a nonsteroidal aromatase inhibitor, NSAI or the aromatase inhibitor alone. This study included individuals with various stages of cancer, ensuring a comprehensive understanding of the treatment’s efficacy across different patient profiles.
Key findings revealed that ribociclib significantly improved invasive disease-free survival rates. Patients receiving the combination therapy had a 3-year survival rate of 90.7 percent compared to 87.6 percent for those on the standard therapy alone. This marked a significant improvement, underscoring the potential of ribociclib in enhancing patient outcomes.
Safety and tolerability were also assessed, with no new adverse events reported. This consistency in safety data offers reassurance to both patients and healthcare providers considering this treatment option. The results are compelling enough to suggest that ribociclib should be considered as an adjuvant therapy for this patient group.
Ribociclib is the second CDK4-6 inhibitor approved by the FDA in early-stage breast cancer, following the approval of abemaciclib. What implications do these findings have for future breast cancer treatments? How might they influence current clinical practice?”
More posts featuring Francisco Esteva.
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
